Volumetric apparatus and calibration
Volumetric apparatus and their calibration.
BIOCHEMISTRY
Dr Pramila Singh
1/9/20247 min read


HSBTE, 2nd Semester, DMLT, Biochemistry, Volumetric apparatuses and their calibration.
Volumetric apparatuses and their calibration
There are wide varieties of volumetric glassware/plastic ware in the biochemistry laboratories, such as beakers, flasks, burettes, Funnels, desiccators, condensers, pipettes, measuring cylinders, etc.
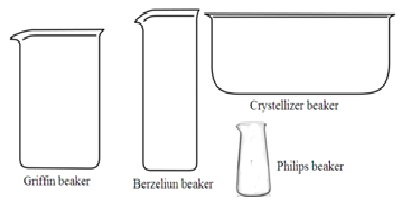
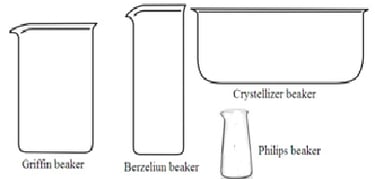
1. Beaker: Beaker is a cylindrical container with a flat bottom. Most of it has a beak (spout) for pouring chemicals. It is made of either borosilicate glass or plastic. It is mainly used for chemicals to hold, stir, mix, and heat during a chemical reaction. It is also used to hold solid or liquid chemicals. The capacity of the beaker may vary from 5ml to 10,000ml. There are four types of beakers.
i. Griffin beakers (lower beaker): Its Height is 40% of its diameter.
ii. Berzeliun beakers (taller beakers): Its height is double to its diameter.
iii. Crystallizer beakers: It does not have measuring marks.
iv. Philips beakers: It is the same as Griffin beakers. But its wall is sloping towards the mouth. The mouth is narrower than the base.
2. Measuring cylinder: (Graduated cylinder or mixing cylinder): It is a narrow cylindrical tall in shape made of plastic or glass. It has a small spout (beak). The beak helps to pour liquid from the measuring cylinder. Mixing cylinders do not have a spout or beak. It has a ground glass joint. Ground glass joint assures air tightness by using the stopper. Ground glass joints in the mixing cylinder also help to connect the mixing cylinder with other apparatus. The capacity of these cylinders varies from 10 ml to 2000 ml. The base of the measuring cylinder or mixing cylinder may be circular or hexagonal. The hexagonal shape gives good stability to the measuring cylinder. The Wall of the measuring cylinder has marked lines that represent the volume of content inside the measuring cylinder.
The measuring cylinder is mainly used to measure the volume of contents present inside the measuring cylinder. It is more accurate and precise to measure liquid than a beaker or flask. Accurate measurement is due to its narrow, cylindrical, and tall shape. However, it is not used to measure liquid in volumetric analysis because a volumetric pipette is more accurate and precise than a measuring cylinder. The measuring cylinder is available in two grades .i.e Grade A and Grade B.
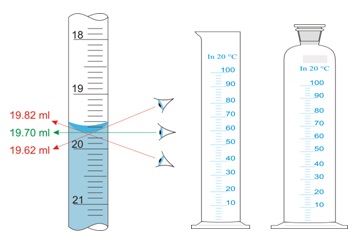
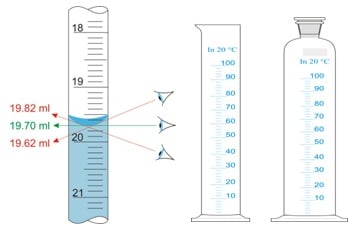
Measurement of volume: The eye level must be at the bottom of the meniscus of liquid. Keeping an eye level above or below of meniscus shall give an inaccurate measurement of the liquid volume.
Reason: The wall of the cylinder attracts molecules of liquid through molecular forces. This molecular force develops a convex or concave shape at the upper surface of the liquid. This meniscus formation of a convex or concave shape depends upon the nature of the liquid. Reading of liquid volume at the bottom part of the concave surface or upper part of the convex surface gives accurate results.
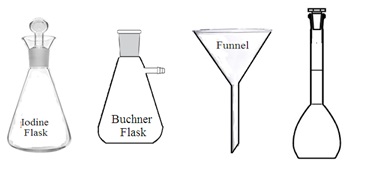
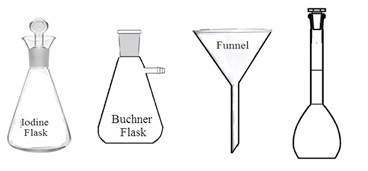
. Flasks: Common features of a laboratory flask are wide vessel (body) with one narrow tubular neck at the top of the flask opening. It is made of either glass or plastic but glass laboratory flasks are preferred. The neck of some laboratory flasks has ground glass joints. Ground glass joints with proper stoppers make it airtight and leakproof. Laboratory flasks are mainly used to hold, contain, collect, and measure liquids. Other processes such as mixing, heating, cooling dissolving, boiling, precipitation, analysis, etc. can also be carried out in laboratory flasks. Its capacity may vary from 25 ml to 5000 ml.
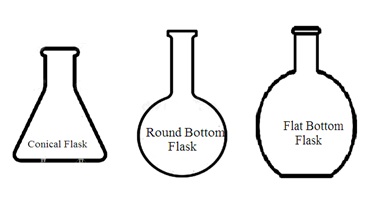
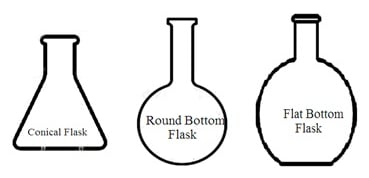
3. Types of flasks: Laboratory flasks are of the following types depending upon their shape and uses: Reaction flasks, round bottom flasks, flat bottom flasks, conical flasks, distillation flasks, evaporating flasks, reagent flasks, cassia flasks, Erlenmeyer flasks, volumetric flask, Dewar flask, powder flask, Retorts, Buchner flask, culture flask, jacketed flask, and Florence flask.
i. Conical flask (Erlenmeyer flask): It is a cone-shaped flask wider at the bottom with a narrow neck at the opening (top). Most conical flasks have ground-glass neck joints to be closed by using a stopper. A conical flask with a grounded glass neck and stopper is also called an iodine flask. They are used to perform a titration and boil the solution. Its narrow neck slows down the evaporation of liquid during titration or boiling.
ii. Round-bottomed flask (Florence flask): It has a round vessel (body) with a long tube-like neck at the opening (top). Some round bottom flasks have to be marked on the neck. This marking helps to measure the total liquid content in the round bottom flask. It also indicates the capacity (volume) of the round bottom flask. Round bottom flasks can withstand high temperatures. It is most suitable for distillation purposes or to boil liquid content. Its neck has grounded glass joints with a stopper. It can also be attached to other glass apparatus through grounded glass joints. These ground glass joints make the round bottom flask airtight and leakproof.
iii. Flat bottom flask: It has a round vessel with a flat bottom. It has a long tube-like neck. It is mainly used to heat liquid.
iv. Volumetric flasks: It has an airtight and leakproof. A volumetric flask is mainly used to make the final volume of liquid with high accuracy.
v. Buchner flask: It is the same as a conical flask. But it has also a side neck. It is made up of thick and chemical-resistant glass. It can withstand pressure differences during vacuum filtration or distillation under reduced pressure.
4. Funnels: A funnel is a tube or pipette-like structure wider at the top and narrow at the bottom. It is made up of stainless steel, aluminum, glass, or plastic. Various types of funnel are used in the laboratory such as filter funnel, dropping funnel, and thistle funnel. Funnels are used to
i. Separate solid from liquid by using a filter funnel.
ii. Separate liquid from the liquid.
iii. Pour liquid, chemical, or solid into the container.
iv. A funnel made of glass or plastic is used in the laboratory.
5. Pipettes: Pipettes are used to measure out or transfer small quantities of liquid. It measures volume in milliliter (ml) or microlitre (µl). They are made of either glass or plastic. There are various types of pipettes.
Measuring pipette: (graduated pipette): It is made of glass and has mark lines throughout the pipette tube. It is used to measure and transfer liquid accurately. The liquid is sucked into the pipette tube by mouth. But it is dangerous. A new automatic pipette is also available. Alternately rubber suction device is used to suck liquid into a pipette tube. It is available in two categories: class-A and class-B. Class-A pipette is more accurate than class–B pipette.
Volumetric apparatus calibration:
Liquid with known density is used to calibrate volumetric apparatus. Mass of liquid present in the volumetric apparatus is determined by using digital balance. or analytical balance. Volume of liquid present in volumetric apparatus is determined by using density of liquid and volume of liquid present in volumetric apparatus. Two parameters are considered. These are :
1. Volumetric apparatus containing volume of liquid and
2. Volume of liquid delivered by the volumetric apparatus deliver.
Note: Density distilled water varies with temperature.
Calibration of volumetric flask:
· Select clean and dry volumetric flask. Rinse it three times by using approximately 2 mL acetone for each rinse. Remove acetone from volumetric flask. Allow the remaining acetone to evaporate from the volumetric flask.
· Weight volumetric flask with stopper using digital balance or analytical balance. Note down their masses.
· Select and measure temperature of distilled water. Fill volumetric flask with distilled water up to mark line on the flask. Note down the weight of volumetric flask containing distilled water. Calculate mass of distilled water inside volumetric flask. Mass of distilled water shall be equal to mass of volumetric flask containing distilled water minus mass of volumetric flak without distilled water.
· Calculate volume of distilled water by using density of distilled water and volume of distilled water. Volume is equal to mass upon density (V= m/d).
· Repeat the procedure three times. Calculate average volume. That is the volume present inside volumetric flask.
Calibration of pipette:
· Select clean and dry pipette.
· Weight an empty beaker of suitable size on digital balance or analytical balance.
· Rinse the selected pipette three times by using distilled water and filling it upto final mark line pipette.
· Fill distilled water upto final mark line on pipette.
· Dispense the distilled water in beaker. Weight beaker with distilled water.Weight of beaker filled with dispensed distilled water minus weight of empty beaker shall be weight of dispensed distilled water.
· Repeat the procedure three times.
· Calculate average volume of dispensed distilled water. This is the volume that will be dispensed by pipette.
Calibration of burette:
· Select clean and dry burette.
· Weight an empty beaker of suitable size on digital balance or analytical balance.
· Rinse the selected burette three times by using distilled water and filling it up to final mark line pipette.
· Select and measure temperature of distilled water. Fill distilled water up to final mark line on burette.
· Dispense the distilled water in beaker. Weight beaker with distilled water. Weight of beaker filled with dispensed distilled water minus weight of empty beaker shall be weight of dispensed distilled water.
· Repeat the procedure three times.
· Calculate average volume of dispensed distilled water. This is the volume that will be dispensed by burette.
Note: Density distilled water depend upon temperature.
Temp. 20 degrees C Density 1.0
Temp 25 degrees C Density 0.9989
Temp 30 degrees C Density 0.9976
