Special Stains
Special stains 1.1 Principle, significance, and interpretation of different types of stains - PAS (Periodic Acid Schiff’s Reagent) - Silver impergnation stain – Reticulin fibre - Ziehl Neelson’s – for AFB and Leprae - Masson’s trichrome stain - Oil Red O – fat - Gram’s stain – Gram +ve and Gram –ve 1.2 Definition of Decalcification 1.3 Process of decalcification 1.4 Various types of decalcifying methods, Their mechanism, advantages, disadvantages, and applications 1.5 Assessment of Decalcification Unit-I
Dr. Pramila Singh
2/18/20249 min read


UNIT I, Special stains
1.1Principle, significance, and interpretation of different types of stains
- PAS (Periodic Acid Schiff’s Reagent)
-Silver impergnation stain–Reticulin fibre
- Ziehl Neelson’s – for AFB and Leprae
- Masson’s trichrome stain
- Oil Red O – fat
- Gram’s stain – Gram +ve and Gram –ve
1.2 Definition of Decalcification
1.3 Process of decalcification
1.4 Various types of decalcifying methods, Their mechanism, advantages, disadvantages, and applications
1.5 Assessment of decalcification
Principle, significance, and interpretation of stains-PAS (Periodic Acid Schiff’s Reagent)
Principle of PAS Stain
PAS stain, or Periodic Acid Schiff's reagent, is a histological staining technique used in pathology and biology to detect the presence of carbohydrates (polysaccharides, glycogen, and mucosubstances) in tissues. The PAS stain is based on the reaction between the reagent and the hydroxyl groups of carbohydrates.
1. Oxidation: Tissue sections are treated with periodic acid, which oxidizes the diol groups of polysaccharides. This creates aldehyde groups.
2. Schiff’s reagent: After oxidation, the tissue is treated with Schiff's reagent, which contains a fuchsin or magenta-colored dye. The aldehyde groups formed in the previous step react with the Schiff's reagent. This results in a stable magenta color.
3. Counter Staining: In some cases, a counterstain (e.g., hematoxylin) may be applied to provide contrast and highlight specific structures.
Significance of PAS Stain:
1. Glycogen detection: PAS stain is particularly useful for highlighting glycogen in tissues. Glycogen is an important storage form of glucose. Its presence or absence can be indicative of various pathological conditions.
2. Mucosubstances: PAS stain is also valuable for identifying mucosubstances. These are glycoproteins or proteoglycans containing carbohydrates. This is especially relevant in the study of tissues with secretory functions.
3. Fungi and Parasites: PAS stain is employed to detect fungi (e.g., Candida) and parasites (e.g., Pneumocystis jirovecii) in tissue sections.
4. Diagnostic Aid: The stain is commonly used in pathology for diagnosing diseases such as glycogen storage diseases, certain types of tumors, and infections involving fungi or parasites.
Interpretation of PAS Stain
1. Magenta Staining: Positive staining appears as a magenta or purple color. It indicates the presence of carbohydrates. This can be seen in structures like glycogen granules and mucin.
2. Absence of Staining: Structures without carbohydrates or with low carbohydrate content will not stain magenta.
3. Counter Staining Effects: If a counterstain is used, it can provide additional information. For example, nuclei may be stained with hematoxylin, allowing for better visualization and interpretation of the tissue architecture.
Principle, significance, and interpretation of Silver impregnation stain – Reticuline fiber
Principle of Silver Impregnation Stain for Reticuline Fibres
Silver impregnation stains are histological techniques that utilize the ability of silver ions to form complexes with certain tissue components. The reticuline stain is used to visualize reticulin fibers. Reticulin fibers are delicate supporting fibers composed of type III collagen. These fibers are abundant in organs like the liver, spleen, lymph nodes, and bone marrow.
Principle of Silver Impregnation Stain for Reticulin Fibres
1. Impregnation: Tissue sections are treated with a silver solution usually a silver nitrate solution. Silver ions form complexes with the reticulin fibers.
2. Reduction: After impregnation, the sections are exposed to a reducing agent (e.g., hydroquinone). This step reduces the silver ions to metallic silver. This leads to the formation of a visible stain along the reticulin fibers.
3. Fixation and Counter Staining: The stained sections are then fixed and may be counterstained with a contrasting color to enhance the visibility of other tissue structures
Significance of Silver Impregnation Stain for Reticulin Fibres:
1. Visualization of Reticulin Fibres: The primary significance of the reticulin stain is its ability to highlight reticulin fibers. It allows for the visualization of the delicate network formed by these fibers in various tissues.
2. Tissue Architecture: Reticulin fibers are an integral part of the connective tissue in organs. Their distribution pattern examination provides information about the architecture and structural organization of tissues.
3. Disease Diagnosis: Changes in reticulin fiber patterns indicate certain pathological conditions. For example, alterations in the reticulin network are observed in conditions such as fibrosis and certain types of tumors.
4. Matching Information: The information obtained from the reticulin stain is often used in conjunction with other staining techniques. It provides a comprehensive understanding of tissue morphology.
Interpretation of Silver Impregnation Stain for Reticulin Fibres
1. Reticulin Network: The presence and pattern of reticulin fibers are the primary aspects to be assessed. Normal tissues show a fine, delicate network. Pathological conditions result in changes such as thickening or disruption of the reticulin framework.
2. Quantitative Analysis: The stain can be used for quantitative analysis. It provides information about the density and distribution of reticulin fibers.
Disease-specific Changes: Different diseases show specific alterations in the reticulin network. For example, cirrhosis of the liver shows a disruption of the normal reticulin structure
Principle, significance, and interpretation of stains - Ziehl Neelson’s – for AFB and Leprae
Principle of Ziehl-Neelson’s Stain
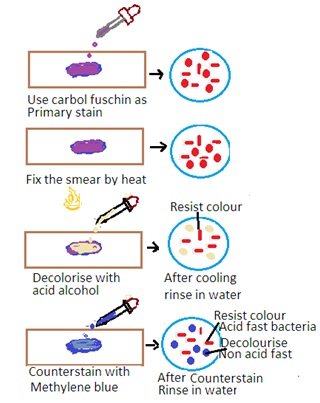
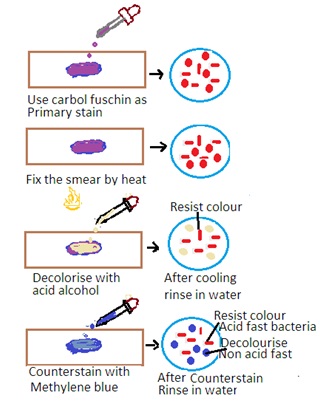
The Ziehl-Neelsen stain is a special staining technique used for the detection of acid-fast bacteria (AFB) such as Mycobacterium tuberculosis (the causative agent of tuberculosis) and Mycobacterium leprae (the causative agent of leprosy). The stain is based on the property of mycobacteria to resist decolorization by acid-alcohol after staining with carbol fuchsin. The principle has the following steps
1. Primary Stain: The specimen is initially stained with carbol fuchsin. Carbon fuscin is a red dye containing phenol. This dye penetrates the mycobacterial cell wall, and the cells retain the stain due to their high lipid content.
2. Heat fixation: The stained specimen is heated to enhance the penetration of the dye into the mycobacterial cells.
3. Decolorization: Acid-alcohol is used as a decolorizing agent. Acid-fast bacteria resist decolorization due to the lipid-rich cell wall. Other bacteria are decolorized.
Counter Staining: The smear is counterstained with a contrasting color, usually methylene blue, to visualize non-acid-fast bacteria.
Write Modified Zeihl-Neelsen Stain for leprae:
A modified Ziehl-Neelsen stain is used for the detection of Mycobacterium leprae. This modification is required due to the low number of bacilli in leprosy lesions. A prolonged staining and careful examination is carried out.
Significance of Ziehl-Neelsen Stain:
1. Specific for Acid-Fast Bacteria: The Ziehl-Neelsen stain is highly specific for acid-fast bacteria, making it a crucial tool for the diagnosis of tuberculosis and leprosy.
2. Rapid Detection: The stain is relatively quick and can provide rapid results, facilitating timely diagnosis and treatment initiation.
3. Field Use: Ziehl-Neelsen stain is used in resource-limited settings. It is very useful in the field due to its simplicity and effectiveness.
Interpretation of Zeihl-Neelsen Stain
1. Acid-Fast Bacteria: Acid-fast bacteria will appear as bright red or pink rods under the microscope. It indicates that they have retained the carbol fuchsin stain.
2. Non-Acid Fast Bacteria: Other bacteria and cellular components will appear blue or green due to the counterstaining with methylene blue.
3. Clinical Correlation: The presence of acid-fast bacilli in clinical specimens is significant and requires correlation with clinical and other laboratory findings for a definitive diagnosis.
Principle, significance, and interpretation of stains Masson’s trichrome stain
Principle of Masson’s Trichrome Stain
Masson's trichrome stain is a histological staining technique used to differentiate and visualize various tissue components, especially collagen and muscle fibers. The stain employs three different dyes to selectively color different structures in tissues. The principle involves the following steps:
1. Weigert’s Iron Hematoxylin: Tissue sections are initially stained with Weigert's iron hematoxylin, which stains cell nuclei and other basophilic structures (e.g., muscle fibers) blue-black.
2. Biebrich Scarlet-Acid Fuchsin: This is the second step and involves staining with Biebrich scarlet-acid fuchsin, which colors muscle fibers and cytoplasmic components red.
3. Phosphotungstic/Phosphomolybdic Acid: Tissue sections are treated with phosphotungstic/phosphomolybdic acid. This serves as a differentiator. This step selectively removes the scarlet color from certain structures and intensifies the blue-black color in others.
4. Aniline Blue: The final step involves staining with aniline blue. This stains collagen and other extracellular matrix components blue.
Significance of Masson’s Trichrome Stain
1. Collagen Visualization: One of the primary applications of Masson's trichrome stain is the visualization of collagen fibers in tissues. Collagen appears blue in the final stained sections.
2. Muscle Fiber Identification: The stain is effective in distinguishing muscle fibers and other tissue components. Thus, it is useful in studies of muscular tissue.
3. Connective Tissue Analysis: Masson's trichrome stain provides information about the distribution and characteristics of connective tissue components. This helps in the study of tissue architecture.
4. Fibrosis Detection: The stain is employed to assess the extent of fibrosis in tissues. Collagen deposition is a hallmark of fibrotic changes.
Interpretation of Masson’s Trichrome Stain
1. Nuclei: Nuclei are stained blue-black due to Weigert's iron hematoxylin.
2. Muscle Fibers: Muscle fibers are stained red by Biebrich scarlet-acid fuchsin.
3. Collagen and Connective Tissue: Collagen fibers and other connective tissue elements appear blue with the aniline blue stain.
4. Fibrosis Assessment: The stain is particularly useful for evaluating the degree of fibrosis in tissues, with increased collagen deposition indicating fibrotic changes.
5. Tissue Architecture: Masson's trichrome stain provides a detailed view of tissue architecture, helping in the identification of different tissue components.
Principle, significance, and interpretation of stains- Oil Red O– fat
Principle of Oil-Red-O-Stain for Fat
Oil Red O is a lipophilic dye. It is commonly used for staining lipids, particularly neutral fats and triglycerides. The principle of Oil Red O staining involves the interaction of the dye with lipid droplets. This results in the staining of fat deposits within cells. The staining procedure typically includes the following steps:
1. Fixation: Tissue sections or cells are fixed using an appropriate fixative to preserve the cellular structure.
2. Incubation with Oil Red O: The fixed specimens are then incubated with Oil Red O. This allows the dye to selectively bind to lipid droplets.
3. Washing: Excess stain is washed away to remove unbound dye.
4. Counter Staining (Optional): In some cases, a counterstain (e.g., hematoxylin) may be applied to provide contrast and enhance the visualization of other cellular structures.
Significance of Oil Red O Stain for Fat:
1. Visualization of Lipid Accumulation: The primary significance of Oil Red O stain is its ability to visualize and identify lipid droplets and fat accumulation within cells and tissues.
2. Detection of Lipid Disorders: The stain is useful in histopathology for identifying abnormal lipid accumulation. This may be associated with various pathological conditions such as fatty liver disease, lipid storage disorders, and certain types of tumors.
3. Research on Adipocytes: Oil Red O is commonly used in research to study adipocytes and lipid metabolism. It helps in assessing the differentiation of adipocytes. It also helps to assess changes in lipid content.
Interpretation of Oil Red O Stain:
1. Red Staining: Lipid droplets within cells will appear red under the microscope. It indicates the presence of fats.
2. Intensity of Staining: The intensity of red staining provides information about the amount of lipid accumulation. More intense staining suggest higher lipid content.
3. Distribution of Lipids: The stain helps in assessing the distribution of lipid droplets within cells and tissues.
4. Comparison with Counterstain (if used): If a counterstain is applied, the contrast between lipid droplets and other cellular structures can be observed.
Note:
· Oil Red O staining is commonly used in the study of adipose tissue, liver sections, and other tissues where lipid accumulation is of interest.
· It's important to note that Oil Red O is not specific for distinguishing between different types of lipids. Additional techniques may be required for detailed lipid characterization.
Principle, significance, and interpretation of stains- Gram’s stain – Gram +ve and Gram –ve
Principle of Gram Stain
Gram stain is a differential staining technique. It is used in microbiology to classify bacteria into two groups based on their cell wall characteristics. The staining process involves several steps:
1. Crystal Violet Staining: The bacterial smear is initially treated with crystal violet, which stains all cells purple.
2. Iodine Treatment: Iodine is applied to form a crystal violet-iodine complex. This step is essential to stabilize the crystal violet stain.
3. Alcohol Decolorization: The smear is treated with alcohol or acetone. This step differentiates bacteria based on their cell wall properties. Some bacteria retain the crystal violet-iodine complex and remain purple (Gram-positive). Others lose the stain and become colorless (Gram-negative).
4. Counter Staining: The final step involves applying a counterstain. Usually, safranin stains the colorless Gram-negative bacteria pink or red. Gram-positive bacteria retain the initial crystal violet stain and appear purple.
Significance of Gram Stain
1. Bacterial Classification: Bacteria are classified into two main groups: Gram-positive and Gram-negative. This classification is based on Gram's staining. Gram staining depends on the cell wall structures of bacteria.
2. Clinical Relevance: The Gram stain result is one of the initial steps to identify and characterize bacterial infections. It helps in treatment decisions. Since Gram-positive and Gram-negative bacteria respond differently to antibiotics.
3. Preliminary Identification: Gram stain provides a quick and preliminary identification of bacteria. It helps microbiologists in selecting further tests for more accurate identification.
4. Shape and Arrangement: Gram stain also provides information about the shape (cocci or bacilli) and arrangement (clusters, chains, pairs) of bacteria.
Interpretation of Gram Bacteria:
1. Gram-Positive Bacteria: These bacteria retain the crystal violet-iodine complex and appear purple under the microscope. Common Gram-positive bacteria include Staphylococcus and Streptococcus.
2. Gram Gram-negative bacteria: These bacteria lose the crystal violet-iodine complex during decolorization and take up the counterstain, appearing pink or red under the microscope. Examples of Gram-negative bacteria include Escherichia coli and Pseudomonas.
3. Cell Morphology and Arrangement: Gram's stain allows for the observation of bacterial cell morphology (round or rod-shaped) and arrangement (singles, pairs, chains, clusters). This is useful in preliminary identification.
4. Clinical Implication: The Gram result guides clinicians in choosing appropriate antibiotics. Gram-positive and Gram-negative bacteria have different cell wall structures. Thus their susceptibilities to antibiotics vary.text here.
Dr Pramila Singh
