Serum Calcium Potassium
Serum Calcium and Potassium Principle and Procedure of Estimation of Serum Calcium Principle and Procedure of estimation of serum potassium Clinical Significance of Calcium/Potassium Estimation
BIOCHEMISTRY
Dr Pramila Singh
11/2/20233 min read
Serum Calcium and Potassium
Principle and Procedure of Estimation of Serum Calcium and Potassium.
Clinical Significance of Calcium/Potassium Estimation.
Principle and procedure of estimation of serum calcium
Principle: Serum calcium estimation requires cresol phthalein complex (CPC) reagent and 8-hydroxyquinoline. CPC reacts with calcium to develop color. 8-hydroxyquinoline is used to block magnesium interference in CPC and calcium reactions.
Reagents
1. Calcium reagent 1: 40 mg
Cresolphthalein complex (CPC) 40 mg in 1.0 mL conc hydrochloric acid
8-hydroxyquinoline: 2.5 gm
Dimethylsulfoxide 100 mL
Distilled water to produce a volume of 1000 L.
2. Calcium reagent 2: Potassium cyanide 500 mg and diethylamine 40 mL in distilled water 960 mL.
3. Calcium standard: Calcium 10 mg/dL.
4. EDTA: 4.0 mg/dL.
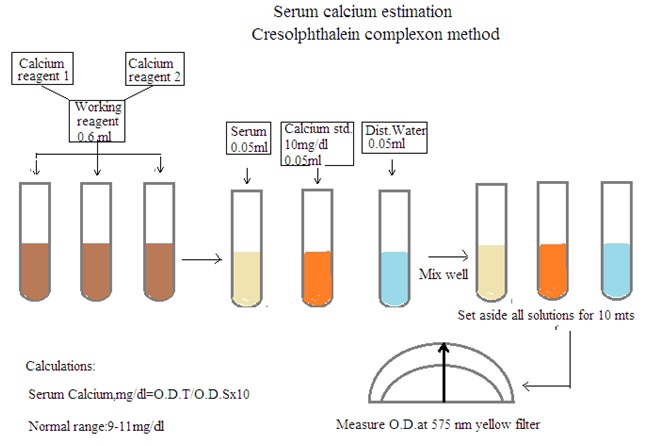
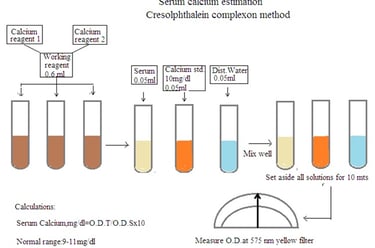
Procedure: Mix calcium reagent 1 and calcium reagent 2 in equal proportion. It is a fresh working reagent. Prepare solution as per below details
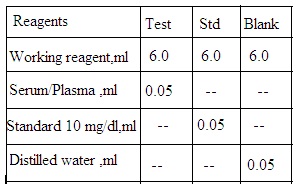
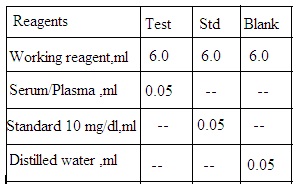
1. Test solution: Working reagent 6 mL, and serum sample 0.05 mL.
2. Standard solution: Working reagent 6 mL and calcium standard 0.05 mL
3. Blank solution: Working reagent 6 mL and distilled water 0.05 mL.
Set aside all solutions for 10 minutes. Measure O.D. using a spectrophotometer at 575 nm (Yellow filter).
Serum calcium mg/dL = O.D. Test/ O.D. Standard X 10.
Normal Range of Serum Calcium: : 8.6 to 10.2 milligram per decilitre (mg/dL) or 2.15 to 2.55 millimoles per liter (mmol/L).
Principle and procedure of estimation of serum potassium
Principle: Blood serum is used to estimate serum estimation. Potassium ions (K+) in serum are measured to estimate serum potassium level. Ion-selective electrodes or colorimetric methods are used to determine serum potassium concentration. Ion-selective electrodes are widely used. It is based on the Nernst equation. It depends upon the potential difference between a potassium-sensitive electrode and a reference electrode.
Potassium is an essential electrolyte in the body. It plays a critical role in various physiological activities like muscle contraction, nerve impulse transmission, normal heartbeat, etc..
It is important to follow the manufacturer’s instructions for specific analyzers.
Normal Range of serum Potassium: 3.8 to 5.6 mEq/L.
Materials and equipment: Serum sample, Potassium ion-selective electrode, Reference electrode, Calibrated Analyzer or Potentiometer, Glassware.
Procedure:
1. Collect blood samples. Centrifuge blood sample to separate serum from blood cells.
2. Load the serum samples and standards into the analyzer.
3. Immerse the ion-selective electrode in the sample and the reference electrode in the reference solution.
4. Electrodes measure the potential difference created by potassium ions in the sample. Converts it into potassium concentration.
Results: Record and report potassium concentration in mmol/L or mEq/L.
Dr Pramila Singh
Clinical significance of calcium/potassium estimation
Serum calcium and potassium
Calcium is a mineral that plays an important role in the formation of bones and teeth. It is also essential for muscle contraction, nerve transmission, blood clotting, and the release of hormones and enzymes. The human body effectively regulates serum calcium levels as per the requirements of the body. Potassium is an essential mineral to maintain the functions of cells, nerve conduction, and muscle contraction. Serum potassium regulates blood pressure and body fluid balance and supports several enzymatic reactions.
Clinical Significance of Calcium: Calcium plays its role in various physiological processes in the body such as bone formation, muscle contraction, blood clotting, enzyme regulation, and nerve functions. Increased or decreased level of calcium in the body indicates abnormal physiological conditions. The following are the significance of serum calcium level estimation.
1. Calcium disorders: Increased level of serum calcium is hypercalcemia and decreased level of serum calcium is hypocalcemia.
2. Hypercalcemia indicates hyperthyroidism, hyperparathyroidism, bone cancer, excess vitamin D intake, and medication side effects. Hypercalcemia leads to fatigue, constipation, kidney stones, cardiac arrhythmias, and several other medical complications.
3. Hypocalcemia indicates hypothyroidism, vitamin D deficiency, chronic kidney disease, calcium absorption disorders, and certain medication side effects. Hypocalcemia leads to muscle cramps, numbness, seizure, cardiac arrhythmias, and tingling in the extremities. Hypocalcemia may be life-threatening.
4. Monitoring chronic conditions: Serum calcium level is estimated to monitor several chronic diseases such as chronic kidney disease, parathyroid disorder, bone disorders like osteoporosis, etc.
5. Vitamin-D Status: Vitamin D increases calcium absorption from the small intestine. Low-level serum calcium indicates Vitamin D deficiency.
Clinical Significance of Potassium: Potassium plays an important role in various physiological conditions of the human body. A low level of serum potassium is called hypokalemia. A high level of serum potassium is called hyperkalemia.
A low level of serum potassium indicates
1. Cardiac effects: Cardiac arrhythmias. It includes tachycardia and fibrillation
2. Muscular effect: Muscle weakness, cramps even paralysis,
3. Gastrointestinal effect: Constipation, intestinal obstruction, and slow bowel motility,
4. Renal effect: Polyurea and prone to kidney stone
5. Metabolic effect: Metabolic alkalosis.
A high level of serum potassium indicates
1. Cardiac effect: Life-threatening cardiac arrhythmias
2. Muscle effect: Muscle weakness and paralysis
3. GIT effect: Nausea, vomiting, and intestinal pain
4. Renal effect: Impaired functioning of the kidney stops potassium excretion and promotes potassium accumulation in the body.
5. Metabolic effect: Metabolic acidosis.
