Serum Amylase, ALP, ACP
Serum Amylase, ALP, and ACP Principle and procedure of estimation of serum amylase. Clinical significance of serum amylase estimation. Principle and procedure of estimation of ALP. Principle and procedure of estimation of ACP. Clinical significance of ALP/ACP estimation.
BIOCHEMISTRY
Dr Parmila Singh
10/11/20235 min read
UNIT III
Dr Pramila Singh
Serum Amylase, ALP, and ACP
3.1 Principle and procedure of estimation of serum amylase
3.2 Clinical significance of serum amylase estimation
3.3 Principle and procedure of estimation of ALP
3.4 Principle and procedure of estimation of ACP
3.5 Clinical significance of ALP/ACP estimation
Serum Amylase, ALP, and ACP
Amylase is an enzyme produced by the pancreas and salivary glands to digest carbohydrates. An increase in serum amylase indicates pancreatic disorders and salivary gland disorders. Alkaline phosphatase (ALP) is an enzyme mainly present in liver cells, bone cells, intestines, and placenta during pregnancy. ALP plays an important role in the metabolism of proteins and fats. A small amount of it is also present in other cells. Increased level of ALP in the blood indicates liver disorder and bone disorder. A low level of ALP indicates nutrient deficiency. It is excreted in the bile to the small intestine and to feces. Acid phosphatase (ACP) is an enzyme produced by the prostate gland in males. It is also released in small amounts by the liver, spleen, and red blood cells. It plays an important role in the metabolism of lipids, proteins, and nucleic acid. ACP is measured to diagnose prostate gland cancer, prostatic hyperplasia, and bone diseases.
Principle and Procedure of Estimation of Serum Amylase
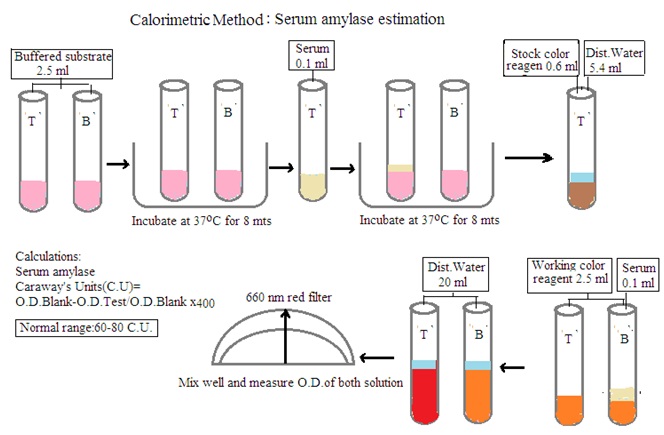
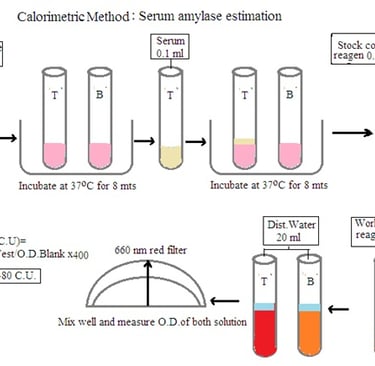
Principle: Amylase acts on starch to produce dextrin and maltose. End products with iodine develop a blue color. The intensity of the blue color is compared with the color developed in the blank. The decrease in blue color intensity is directly proportional to the amylase concentration in the sample.
Reagents:
Buffered substrate: 2.66 gm
Stock color reagent: 0.3567 gm potassium iodate, 4.5 gm potassium iodide, and 0.9 mL concentrated hydrochloric acid in 100 mL distilled water.
Working color reagent: 1:10 ratio of stock color reagent and distilled water.
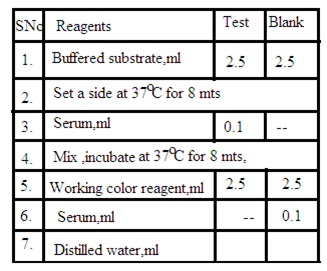
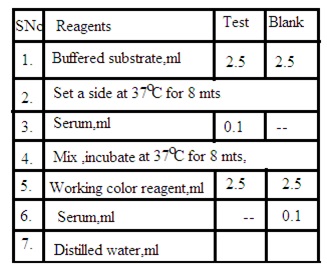
Procedure: Prepare test solution and blank solution as per the below details
Test solution: Mix 2.5 mL buffered substrate and 0.1 mL Serum sample. Set aside for 8 minutes at 37 degrees C. Add 2.5 mL working color reagent and 20 mL distilled water.
Blank solution: Mix 2.5 mL buffered substrate, 2.5 mL working color reagent, 0.1 mL serum sample, and 20 mL distilled water.
Measure the O.D. of both solutions and calculate the amount of serum amylase by using the following formula
O.D. Blank solution- O.D. Test solution/ O.D. Blank solution X 400.
Clinical significance of serum amylase estimation:
Serum amylase estimation helps to diagnose and monitor several medical conditions such as:
Pancreatic disorder: An increase in serum amylase level indicates pancreatic disorders such as acute pancreatitis, chronic pancreatitis, and pancreatic cancer.
Salivary gland disorders: an increase in serum amylase indicates salivary gland stone or salivary gland infection.
Kidney disease: IncreaseD serum amylase indicates acute renal failure. The exact mechanism is not known.
Abdominal conditions: An increase in serum amylase indicates bowel obstruction, peptic ulcer, and GIT perforation.
Disease monitoring: Decrease in serum amylase indicates the effectiveness of treatment of above said diseases.
Principle and procedure of estimation of Alkaline phosphatase (ALP)
Principle: P-nitrophenyl phenyl (PNP) is a colourless reagent. Enzyme alkaline phosphatase breaks PNP to release phosphate group and P-nitrophenol. P-nitrophenol is colourless in acidic pH. P-nitrophenol forms yellow-coloured P-nitro phenoxide ions in alkaline pH. The intensity of the yellow colour will be proportional to the presence of ALP in the serum sample. The intensity of the yellow colour in the serum sample is measured by spectrophotometry at 405 nm (violet filter).
Normal Range: In adults: 20 to 90 IU, In children: 93 to 221 IU/
Reagents:
1. Amino methyl propanol buffer (AMP) pH 10.3
2. P-nitrophenyl phosphate powder
3. Magnesium chloride reagent 30 mg/dL.
4. P-nitrophenol standard.
5. 0.25N Sodium Hydroxide.
Prepare fresh reagent to be used.
Procedure: Prepare test and blank solution as per the below details
Test Solution: 2.2 ml AMP and 0.2 mL working substrate. Mix and set aside for 5 minutes. Add 0.1 mL serum sample. Mix and set aside for 15 minutes at 37 degrees C. Add 3 mL 0.25N Sodium hydroxide.
Blank solution: 2.2 ml AMP and 0.2 mL working substrate. Mix and set aside for 5 minutes. Add 0.1 mL serum sample. Add 3 mL 0.25N Sodium hydroxide. Read the intensity of the test solution against the blank solution at 405 nm. Calculate the amount of ALP in the IU unit by using a standard graph.
Principle and procedure of estimation of Acid phosphatase (ACP)
Principle: Acid phosphatase converts P-nitrophenyl (PNP) to nitrophenol at pH 4.9. Citrate Buffer is used to maintain pH. Nitrophenol colour intensity is measured after diluting it with 0.1N Sodium hydroxide. P-nitrophenol is colourless in acidic pH. P-nitrophenol forms yellow-coloured P-nitrophenoxide ions in alkaline pH. The intensity of the yellow colour will be proportional to the presence of ACP in the serum sample. This colour intensity is due to acid phosphate in serum from all tissues of the body. This gives the result of total acid phosphatase in the blood sample.
Tartrate reagent is used to measure Prostatic acid phosphatase. Tartrate reagent inhibits prostatic fraction in blood samples. Here colour intensity in the serum sample develops due to Non-prostatic acid phosphatase. Prostatic phosphatase is calculated by subtracting the non-prostatic acid phosphatase value from the total acid phosphatase value.
Normal Range: Total acid phosphate: 0.9 to 12 IU. Prostatic acid phosphate: 0 to 4.0 IU.
Reagent:
1. Citrate buffer pH 4.9
2. Substrate solution (P-nitrophenyl PNP) 0.4 gm in 100 ml D. Water.
3. 0.1N Sodium hydroxide.
4. Tartarate solution: 1.5 gm tartaric acid in citrate buffer pH 4.9
5. Nitrophenol standard 4.173 gm/dL.
6. Fresh working substrate: Mix equal parts of reagent 1 (citrate buffer) and reagent 2 (Substrate solution). It is stable at 2 to 8 degrees C for 12 hours.
Procedure: Collect fresh serum samples free from hemolysis. Prepare followings solution
1. Total ACP test solution: Mix working solution 1 mL, and serum sample 0.2mL. Set aside for 30 minutes at 37 degrees C. Add 0.1N Sodium hydroxide.
2. Non-prostatic ACP test solution: Mix working solution 1 mL, and Tartarate solution 0.2 mL. Set aside for 5 minutes at 37 degrees C. Add 0.2 mL serum sample. Set aside for 30 minutes at 37 degrees C. Add 0.1N Sodium hydroxide.
3. Blank solution: Mix working solution 1mmL and 0.1 N Sodium hydroxide.
Measure O.D. of Total ACP test solution and Non-prostatic ACP test solution against blank solution. Calculate the amount of total ALP and non-prostatic ALP using a standard graph.
Clinical significance of ALP/ACP estimation
Increased level of serum ALP indicates the following disorders
1. Liver disorders: Increased level of serum ALP/ACP indicates liver disorders such as hepatitis, cirrhosis, and liver tumour.
2. Bone disorder: Increased serum ALP/ACP indicates increased bone cell activity, osteomalacia and healing fracture. A decreased ALP level indicates osteoporosis.
3. Pregnancy: During pregnancy, serum ALP/ACP increases. Measuring ALP/ACP level helps to assess the progress of pregnancy and placental function.
4. Gastrointestinal disorder: Certain GIT diseases such as celiac disease, intestinal obstruction, gall bladder stone and obstructive Jaundice.
Dr Pramila Singh
