Immunolopathology and Cytology Practical
HSBTE DMLT IVth Semester Immunolopathology and Cytology Practical
Dr. Pramila Singh
3/16/202412 min read
HSBTE IVth Semester Immunopathology and Cytology Practicals
Experiment No 1
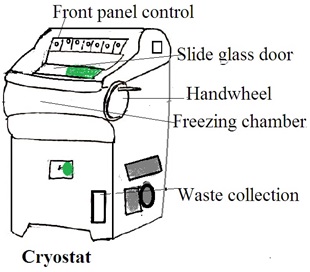
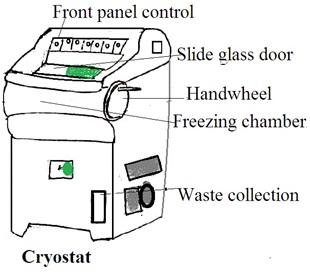
Aim: Demonstration of cryostat (brochures and charts can be used)
Theory: A cryostat is a device or system designed to maintain extremely low temperatures, near absolute zero (0 Kelvin or -273.15 degrees Celsius). This low temperature induces specific physical or chemical behaviors in materials. Or, this low temperature is low enough for the operation of specialized equipment.
It is used in various scientific and industrial applications where very low temperatures are required.
The Components of a Cryostat
1. Cryogenic Vessel (Dewar flask): This is a container that holds cryogenic fluids, such as liquid nitrogen or liquid helium. The dewar flask is a well-insulated double-walled vessel to minimize heat transfer and maintain low temperatures.
2. Thermal insulation: Cryostats are equipped with advanced thermal insulation. Common insulation materials include vacuum insulation panels, multi-layered reflective shields, and other high-efficiency insulating materials.
3. Temperature Control System: A temperature control system is a refrigeration unit. It is used to cool the interior of the cryostat to extremely low temperatures. It uses liquid nitrogen, liquid helium, or closed-cycle refrigerators.
4. Cryogenic Fluid Circulation System: In some cryostats, there is a system for circulating and managing the flow of cryogenic fluids to maintain a constant low temperature.
5. Sample Holder: The sample holder or stage is to hold the sample for cooling.
6. Vacuum Pump: The cryostat may have a vacuum pump to maintain a low-pressure environment in the insulating space. It reduces heat transfer through conduction.
Application: Cryostats are used for various applications, such as the preparation of frozen tissue sections for histological and pathological studies. They are particularly useful for preserving the natural state of tissues without the need for fixation and paraffin embedding.
Working Process
1. Cooling the Dewar flask (Cryogenic Vessel): The refrigeration system cools the inner Dewar flask. It lowers the temperature of the entire cryostat.
2. Sample Insertion: Aloow cryostat reaches the desired low temperature. Insert the sample is carefully into the sample holder or stage.
3. Thermal Shield Operation: The thermal shield maintains a stable temperature. It reduces heat influx from the external environment.
4. Temperature Control: The temperature control system continuously monitors and adjusts the temperature of the cryostat. It ensures the sample remains at the desired low temperature.
5. Experimentation and Analysis: Researchers perform experiments or analyses with the sample at extremely low temperatures. Such as studying material properties, conducting spectroscopy, or examining biological specimens.
Experiment No 2
AIM: Processing of tissue for frozen section
Theory: Frozen section cutting is a rapid diagnostic technique used in pathology. This method allows for the quick examination of tissue samples. Frozen section cutting is particularly useful in surgeries where immediate information about the nature of a tissue lesion is required. For example, it may be employed during cancer surgeries. The surgeon determines based on a report about the need to remove additional tissue, perform further procedures, or if the current resection is sufficient.
It's important to note that frozen section analysis provides quick results. However, the final and more detailed pathology report is obtained through traditional formalin-fixed, paraffin-embedded tissue processing. But this process takes more time but provides a more comprehensive analysis
Procedure: The following steps are followed in the frozen section cutting process:
1. Sample Collection: A small piece of tissue is removed during surgery for analysis. The tissue sample should be representative of the lesion or area of interest.
2. Tissue Embedding: The tissue is embedded in a special compound called OCT (optimal cutting temperature) compound or other freezing mediums. OCT helps to preserve the tissue's cellular structure.
3. Freezing: The embedded tissue is rapidly frozen using a cryostat or a freezing microtome. A cryostat is a device that maintains extremely low temperatures. Cryostat allows precise sectioning of frozen tissues.
4. Sectioning: The frozen tissue block is then sectioned into thin slices (usually 5 to 10 micrometers thick) using a microtome within the cryostat. The sections are collected on glass slides.
5. Staining: The frozen tissue sections are quickly stained with special dyes. It highlights cellular structures and details. Common stains used include hematoxylin and eosin (H&E), which provide contrast between the cell nuclei (hematoxylin) and the cytoplasm (eosin).
6. Examination: The stained sections are examined under a microscope by a pathologist. The pathologist looks for characteristic features that can help in making a preliminary diagnosis.
7. Reporting: Based on the findings, the pathologist provides a report.
Experiment No 3
AIM: Staining and mounting of the frozen section using H&E stain (rapid method).
Theory: Hematoxylin and eosin (H&E) staining is a widely used histological staining method. It provides contrast between different structures within tissues. The staining process involves two main components: hematoxylin and eosin. Hematoxylin stains cell nuclei blue, and eosin stains cytoplasm and extracellular structures pink. The conventional H&E staining process usually takes several hours. However, if a rapid staining is essential then a modified or rapid H&E staining process is used.
Procedure: Preparation of the Hematoxylin and eosin (H&E) stain
1. Hematoxylin solution
· Hematoxylin 1 gm
· Absolute Alcohol 10mL
· Aluminium Potassium Sulphate 20 gm
· Distilled Water 200 mL
· Mercuric Oxide 0.5 gm
2. Eosin Aqueous 5% in water.
The following steps are followed for a H&E stain.
1. Fixation: The tissue is initially fixed using a fixative solution (such as formalin) to preserve its structure.
2. Dehydration: The tissue is dehydrated through a series of alcohol solutions of increasing concentration.
3. Clearing: The tissue is cleared using a clearing agent (usually xylene). It makes the specimen transparent.
4. Staining: The tissue is quickly dipped in hematoxylin. It stains the nuclei. Then tissue is dipped in eosin. It stains the cytoplasm.
Mounting of frozen section.
Mounting frozen sections is a step in the preparation of tissue samples for microscopic examination. The following are steps of the process of mounting frozen sections:
1. Tissue Transfer: The tissue sections are carefully transferred onto glass slides. The slides are usually pre-cooled to enhance the adhesion of the tissue sections.
2. Mounting Medium: A mounting medium is applied to the surface of the glass slide. The mounting medium secures the tissue sections in place and protects the morphology of the tissue in further steps. The mounting medium contains glycerol or a synthetic resin.
3. Flattening and Spreading: Place a cover slip gently on the tissue sections present on a glass slide. The cover slip flattens and spreads the tissue on the glass slide. This helps minimize distortion and ensures even contact between the tissue and the slide.
4. Removal of Excess Medium: Wiped away excess mounting medium carefully present around the edges of the coverslip.
5. Microscopic Examination: Once the mounting medium has been set and the tissue sections are firmly attached to the slide, the slides are ready for microscopic examination.
Experiment No 4
Aim: To stain paraffin-embedded section for the demonstration of reticulin fibers by Silver impregnation stain.
Principle: Silver impregnation stains are histological techniques that utilize the ability of silver ions to form complexes with certain tissue components. The reticuline stain is used to visualize reticulin fibers. Reticulin fibers are delicate supporting fibers composed of type III collagen. These fibers are abundant in organs like the liver, spleen, lymph nodes, and bone marrow.
Procedure:
1. Impregnation: Tissue sections are treated with a silver solution usually a silver nitrate solution. Silver ions form complexes with the reticulin fibers.
2. Reduction: After impregnation, the sections are exposed to a reducing agent (e.g., hydroquinone). This step reduces the silver ions to metallic silver. This leads to the formation of a visible stain along the reticulin fibers.
3. Fixation and Counter Staining: The stained sections are then fixed and may be counterstained with a contrasting color to enhance the visibility of other tissue structures
Significance of Silver Impregnation Stain for Reticulin Fibres:
1. Visualization of Reticulin Fibres: The primary significance of the reticulin stain is its ability to highlight reticulin fibers. It allows for the visualization of the delicate network formed by these fibers in various tissues.
2. Tissue Architecture: Reticulin fibers are an integral part of the connective tissue in organs. Their distribution pattern examination provides information about the architecture and structural organization of tissues.
3. Disease Diagnosis: Changes in reticulin fiber patterns indicate certain pathological conditions. For example, alterations in the reticulin network are observed in conditions such as fibrosis and certain types of tumors.
4. Matching Information: The information obtained from the reticulin stain is often used in conjunction with other staining techniques. It provides a comprehensive understanding of tissue morphology.
Interpretation of Silver Impregnation Stain for Reticulin Fibres
1. Reticulin Network: The presence and pattern of reticulin fibers are the primary aspects to be assessed. Normal tissues show a fine, delicate network. Pathological conditions result in changes such as thickening or disruption of the reticulin framework.
2. Quantitative Analysis: The stain can be used for quantitative analysis. It provides information about the density and distribution of reticulin fibers.
3. Disease-specific Changes: Different diseases show specific alterations in the reticulin network. For example, cirrhosis of the liver shows a disruption of the normal reticulin structure.
Experiment No 5
Aim: To stain paraffin-embedded section using Oil Red “O” stain.
Principle: Oil Red O is a lipophilic dye. It is commonly used for staining lipids, particularly neutral fats and triglycerides. The principle of Oil Red O staining involves the interaction of the dye with lipid droplets. This results in the staining of fat deposits within cells.
Procedure: The staining procedure typically includes the following steps:
1. Fixation: Tissue sections or cells are fixed using an appropriate fixative to preserve the cellular structure.
2. Incubation with Oil Red O: The fixed specimens are then incubated with Oil Red O. This allows the dye to selectively bind to lipid droplets.
3. Washing: Excess stain is washed away to remove unbound dye.
4. Counter Staining (Optional): In some cases, a counterstain (e.g., hematoxylin) may be applied to provide contrast and enhance the visualization of other cellular structures.
Significance of Oil Red O Stain for Fat:
1. Visualization of Lipid Accumulation: The primary significance of Oil Red O stain is its ability to visualize and identify lipid droplets and fat accumulation within cells and tissues.
2. Detection of Lipid Disorders: The stain is useful in histopathology for identifying abnormal lipid accumulation. This may be associated with various pathological conditions such as fatty liver disease, lipid storage disorders, and certain types of tumors.
3. Research on Adipocytes: Oil Red O is commonly used in research to study adipocytes and lipid metabolism. It helps in assessing the differentiation of adipocytes. It also helps to assess changes in lipid content.
Interpretation of Oil Red O Stain:
1. Red Staining: Lipid droplets within cells will appear red under the microscope. It indicates the presence of fats.
2. Intensity of Staining: The intensity of red staining provides information about the amount of lipid accumulation. More intense staining suggests higher lipid content.
3. Distribution of Lipids: The stain helps in assessing the distribution of lipid droplets within cells and tissues.
4. Comparison with Counterstain (if used): If a counterstain is applied, the contrast between lipid droplets and other cellular structures can be observed.
Experiment No 6
Aim: Preparation of Kaiserling's solution I and II for museum specimens.
Kaiserling proposed museum techniques in 1897. Three different solutions are used in the original techniques. These are
1. Kaiserling’s fluid-I: It is a fixing solution. Its components are
Formalin 400 mL,
Potassium Nitrate 30 gm
Potassium Acetate 60 gm
Tap water filtered 2,000 mL.
2. Kaiserling’s fluid-II:. It contains 80% v/v ethyl alcohol. It is for restoring color in an emergency.
3. Kaiserling’s fluid-III: It is mounting media for the biological specimen Its components are
Glycerine 300 mL
Sodium Acetate 100 gm
Formalin 5 mL
Tap water filtered 1000 mL.
Experiment No 7
Aim: Preparation of various mounting reagents for museum specimens.
Theory: Mounting reagents preserve and protect the specimens for long-term storage and display. The following are some common mounting reagents and their preparation methods:
1. Glycerin Gelatin: Glycerin gelatin is a commonly used mounting medium for preserving delicate specimens like insects.
Ingredients:
Gelatin powder
Glycerin
Water
Preparation: Dissolve gelatin powder in warm water according to the manufacturer's instructions. Add glycerin to the gelatin solution and mix well. Allow the mixture to cool and solidify before use. Warm slightly if necessary to liquefy for application.
2. Canada Balsam: Canada balsam is a resinous substance used for mounting microscope slides, particularly for botanical specimens.
Ingredients:
Canada balsam resin
Xylene or toluene (as a solvent)
Preparation: Place Canada balsam resin in a clean glass container. Add an appropriate amount of xylene or toluene to dissolve the resin. The concentration may vary depending on the desired viscosity of the mounting medium. Stir the mixture until the resin is completely dissolved. Store in a tightly sealed container to prevent evaporation.
3. Entellan: Entellan is a synthetic resin used for mounting microscope slides, particularly for histological specimens.
Ingredients:
Entellan or similar synthetic resin
Xylene or toluene (as a solvent)
Preparation: Place Entellan resin in a clean glass container. Add xylene or toluene to dissolve the resin, following the manufacturer's recommended proportions. Stir the mixture until the resin is completely dissolved. Store in a tightly sealed container away from light and heat.
4. Polyvinyl Alcohol (PVA): PVA is commonly used for mounting zoological specimens, especially in wet collections.
Ingredients:
Polyvinyl alcohol powder
Water
8. Preparation: Dissolve polyvinyl alcohol powder in warm water, stirring continuously until completely dissolved. Allow the solution to cool and filter it through a fine mesh to remove any impurities. Store the PVA solution in a tightly sealed container, away from light and heat.
Experiment No 8
Aim: Processing and Labeling of various museum specimens.
Processing and labeling various museum specimens involves several steps to ensure their proper preservation, documentation, and accessibility for research and display purposes. The following are the processing and labeling of different types of museum specimens:
1. Collection and Initial Documentation: Specimens are collected following ethical guidelines and relevant permits. Basic information such as date, location, collector, habitat, and associated data are recorded on field tags or collection notebooks.
2. Cleaning and Preparation: Specimens are cleaned to remove dirt, debris, and excess tissue, using appropriate methods for each specimen type (e.g., brushing, washing, or chemical treatments). Preparation may involve dissection, drying, or other techniques to stabilize the specimen.
3. Mounting and Storage: Specimens are mounted or stored using suitable methods depending on their type. Storage containers should be acid-free and provide adequate protection from light, pests, and environmental fluctuations.
4. Labeling: Each specimen should be labeled with essential information to ensure its proper identification and documentation:
Scientific name (genus and species).
Collector(s) name(s) and collection number.
Collection date and location (including GPS coordinates if available). Any relevant additional information.
Labels should be printed or written using archival-quality materials to ensure longevity. Labels are typically placed adjacent to or beneath the specimen, ensuring they do not obstruct viewing or damage the specimen.
Experiment No 9
Aim: Demonstration and care of autopsy instruments
Autopsy instruments
Autopsy instruments cover a wide range of tools used during post-mortem examinations. The following are some commonly used autopsy instruments:
1. Scalpel: A small, sharp knife used for making incisions.
2. Autopsy Needle: Used for suturing incisions during the autopsy.
3. Scissors: Various types, including dissecting scissors for cutting tissue.
4. Y-shaped Autopsy Scissors: Specifically designed for opening the cranial cavity.
5. Forceps: Grasping and holding instruments; e.g., tissue forceps or dissecting forceps.
6. Bone Forceps: For handling and grasping bones.
7. Surgical Blades: Used in conjunction with a handle (scalpel) for cutting.
8. Bone Saw: Used for cutting through bone during autopsies.
9. Rib Shears: Specifically designed for cutting through ribs.
10. Post-Mortem Hammer: Used for disarticulating joints and breaking bones.
11. Y-shaped Autopsy Scissors: Specifically designed for opening the cranial cavity.
12. Raspatories and Elevators: Tools for lifting tissues or bones during dissection.
13. Trocar and Cannula: Used for aspirating fluids from body cavities.
14. Tissue Sampling Tools: Such as biopsy punches or needles for collecting samples.
15. Mallet: Used for delicate bone work or dislodging organs.
16. Organ Bags: Used to contain and transport organs during the examination.
17. Specimen Containers: Containers for preserving and transporting tissue samples.
18. Ruler/Calipers: Used for measuring dimensions of organs or injuries.
19. Dissecting Table: The surface on which the autopsy is performed.
20. Gloves: Protective gear worn by autopsy personnel.
21. Autopsy Apron/Gown: Protective clothing worn during autopsies.
Care and maintenance of autopsy instruments:
Proper care and maintenance of autopsy instruments are essential to ensure accurate and reliable results during post-mortem examinations. The following are some general guidelines for maintaining autopsy instruments:
1. Cleaning:
Immediate Cleaning: Instruments should be cleaned immediately after each use to prevent the drying of tissues and fluids.
Use of Enzymatic Cleaners: Autopsy instruments are soaked in enzymatic cleaners to break down organic materials. Follow the manufacturer's instructions for proper dilution and soaking time.
Ultrasonic Cleaning: Some instruments can be cleaned using ultrasonic cleaners to remove debris from hard-to-reach areas.
2. Disinfection:
Chemical Disinfection: Autopsy instruments are disinfected after cleaning. Use appropriate disinfectants recommended for medical instruments, and follow the manufacturer's instructions.
Sterilization: Some instruments may require sterilization. Autoclaving is a common method. However, all instruments cannot bear high temperatures and pressure. For these check manufacturer guidelines.
3. Drying: Ensure instruments are thoroughly dried after cleaning and disinfection. It prevents corrosion. Use a clean, dry cloth or air-dry the instruments.
4. Storage: Store instruments in a dry, clean environment to prevent corrosion. Use instrument trays or containers specifically designed for storage.
Avoiding Contact: Store instruments in a way that avoids contact with other metallic objects to prevent scratches and damage.
5. Regular Inspection:
Visual Inspection: Regularly inspect instruments for signs of wear, damage, or corrosion. Replace or repair any instruments that show signs of deterioration.
Sharpening: Sharp instruments like scalpels and scissors should be regularly inspected and sharpened as needed.
Instrument-Specific Care:
Follow Manufacturer Guidelines: Adhere to the manufacturer's guidelines for care and maintenance specific to each type of instrument.
9. Documentation:
Record Keeping: Maintain a log of instrument usage, cleaning, disinfection, and any maintenance or repairs performed. This documentation can be useful for quality control and audit purposes.
Training and Education:
Staff Training: Ensure that staff members are properly trained on the care and maintenance of autopsy instruments. Follow established protocols and guidelines.
Dr Pramila Singh
