Fungal Infection
Collection, processing of sample for fungal infection in Skin, Nail and Hair - KOH preparation - LCB (Lactophenol cotton blue) - India ink 2.2 Fungal Culture media SDA (sabouraud's dextrose agar) with and without antibiotics CMA (Com meal agar) BHI (Brain Heart Infusion)
Dr Pramila Singh
3/1/202415 min read
Collection, processing of sample for fungal infection in Skin, Nail and Hair
- KOH preparation
- LCB (Lactophenol cotton blue)
- India ink
2.2 Fungal Culture media. SDA (Sabouraud's dextrose agar) with and without antibiotics. CMA (Com meal agar).
BHI (Brain Heart Infusion).
Collection and processing of samples for fungal infection in the Skin
Collecting and processing samples to diagnose fungal infections of the skin involves careful and sterile procedures. This ensures accurate results. Skin samples are collected for laboratory analysis to identify the causative fungi and guide appropriate treatment.
Collection of samples for fungal infection in Skin:
It's essential to transport samples promptly to the laboratory and communicate relevant clinical information to ensure accurate and timely diagnosis. The following steps are followed to collect skin samples:
1. Preparation: Wash hands thoroughly and wear sterile gloves to prevent contamination. Explain the procedure to the patient, including the importance of proper sample collection for an accurate diagnosis. Clean the affected area of the skin with an antiseptic solution to minimize surface contamination.
2. Collection Method:
Scrapings: Gently scrape the affected skin to collect scales, crusts, or debris by using a sterile scalpel or the edge of a glass slide. Collect material from the advancing edge of the lesion. In this part, fungi are more concentrated.
Skin Swab: Alternatively, a sterile cotton swab can be rolled over the affected area to collect superficial material. Swabs are particularly useful for moist lesions.
Hair Plucking: If the infection involves hair follicles, pluck a few hairs with their roots using sterile forceps.
Nail Clippings: For suspected nail infections (onychomycosis), clip a portion of the affected nail, including the nail plate and any debris beneath it.
3. Multiple Samples: Collect multiple samples from different lesions if present. This can improve the chances of detecting the fungal pathogen, especially in cases of patchy infections.
Processing of Sample for Fungal Infection in Skin
1. Direct Microscopic Examination: Place a portion of the collected material on a glass slide and add a drop of 10-20% potassium hydroxide (KOH). Gently heat the slide to dissolve keratin and other debris. Cover the specimen with a cover slip and examine it under a microscope. Fungal elements, such as hyphae, spores, or yeast cells, can be observed.
2. Fungal Cultures: Inoculate a portion of the collected material onto appropriate fungal culture media, such as Sabouraud dextrose agar. Incubate the cultures at the appropriate temperature (usually 25-30°C for most dermatophytes) and monitor for fungal growth.
3. Histopathological Examination: A biopsy of the affected skin may be performed for more invasive infections or when the diagnosis is unclear. The tissue is fixed, sectioned, and stained for histopathological examination.
4. Molecular Methods: Polymerase chain reaction (PCR) and other molecular techniques may be employed for more rapid and specific identification of fungal species. This is particularly useful in cases where conventional methods are inconclusive.
5. Antifungal Susceptibility Testing: In some cases, especially when dealing with recurrent or resistant infections, antifungal susceptibility testing may be performed to guide treatment decisions.
Collection and processing of samples for fungal infection in the Nail.
The collection and processing of samples for fungal infection in the nail is known as onychomycosis. It involves careful procedures to obtain material that can be examined microscopically or cultured for the presence of fungi. The following steps are followed to collect and process nail samples for fungal infection.
1. Patient Preparation: Explain the procedure to the patient, including the importance of proper sample collection for an accurate diagnosis. Ensure the patient's hands or feet are clean, and the nails are dry.
2. Collection Tools: Use a pair of sterile nail clippers or a sterile scalpel for sample collection. Sterilize the tools before use to prevent contamination.
3. Nail Sampling: Select the affected nail(s) for sampling. Ideally, choose a nail that shows clinical signs of infection, such as discoloration, thickening, or deformation. Trim the nail as close to the affected area as possible. Ensure that both the free edge and the material from beneath the nail are collected. If the infection is suspected in the nail bed, collect material from the subungual area using a sterile curette or scalpel.
4. Sample Size: Collect an amount of nail material sufficient for both microscopic examination and culture. The collected material should include nail fragments and debris.
5. Handling and Packaging: Place the collected nail material into a sterile, leak-proof container. This step is crucial to avoid contamination during this process. Label the container with patient information, including name, date of birth, and the site of sample collection.
6. Microscopic Examination: A part of the collected sample can be directly examined under a microscope after mounting it in a solution like potassium hydroxide (KOH). This helps in visualizing fungal elements such as hyphae, spores, or pseudohyphae. Microscopic examination can provide a rapid diagnosis but may not identify the specific fungal species.
7. Culture: The remaining portion of the sample can be cultured on specific fungal media, such as Sabouraud dextrose agar, to isolate and identify the causative fungi. Incubate the culture at an appropriate temperature (usually 25-30°C) and monitor it for fungal growth over several weeks.
8. Identification: Once fungal colonies appear on the culture, they can be identified through macroscopic and microscopic characteristics, biochemical tests, or molecular methods.
9. Identification: Once fungal colonies appear on the culture, they can be identified through macroscopic and microscopic characteristics, biochemical tests, or molecular methods. Identification is crucial for determining the appropriate antifungal treatment.
10. Additional Tests (if needed): In some cases, additional tests like polymerase chain reaction (PCR) or fungal DNA sequencing may be required for precise identification, especially when traditional methods are inconclusive.
11. Reporting: Report the findings to the healthcare provider, including the identification of the fungal species and any treatment recommendations.
Proper sample collection and processing are essential for accurate diagnosis and effective management of fungal nail infections. Adhering to sterile techniques, avoiding contamination, and thorough communication between the laboratory and healthcare provider contribute to successful diagnostic outcomes.
Collection and processing of samples for fungal infection in the Hair
Collecting and processing samples for the diagnosis of fungal infections in the hair requires obtaining specimens from affected areas to identify the causative agent. Proper collection and processing of samples are essential for accurate diagnosis and identification of the causative agent in hair-related fungal infections. The following are general guidelines on the collection and processing of samples for hair-related fungal infections.
Collection of samples for fungal infection in the Hair:
1. Skin and Hair Scrapings: Collect scales, debris, or hair fragments from the affected area using a blunt scalpel or the edge of a glass slide. Ensure that the sampling is not traumatic to the patient.
2. Hair Plucking: If the infection involves the hair shaft, pluck several infected hairs from the root. Include both diseased and healthy-looking hairs to increase the chances of identifying the fungal agent.
3. Epilated Hairs: Hairs can be removed using forceps or tweezers. Ensure that the root and surrounding tissue are included. This method is suitable for cases where hair is easily removable.
4. Cotton Swab Technique: Rub a sterile cotton swab over the affected area to collect debris and scales. This method is suitable for superficial infections and when other methods may be impractical.
Processing of Samples
1. Direct Microscopy: Place the collected material on a glass slide and add a drop of potassium hydroxide (KOH) solution. Cover with a coverslip and gently heat. This process helps in clearing keratin and visualizing fungal elements under a microscope. Fungal hyphae and spores can be observed.
2. Fungal Cultures: Inoculate a portion of the collected material onto appropriate fungal culture media (e.g., Sabouraud agar). Incubate the culture at appropriate temperatures for the suspected fungal pathogen (e.g. room temperature or 37°C for dermatophytes). Cultures allow for the growth and identification of the causative fungus.
3. Histopathological Examination: In cases of severe or deep-seated infections, a biopsy of the affected area may be necessary. The biopsy specimen is fixed, embedded in paraffin, sectioned, and stained with special fungal stains (e.g., Periodic Acid-Schiff or Gomori Methenamine Silver). Histopathology helps in identifying fungal elements and assessing tissue involvement.
4. Molecular Techniques: Polymerase chain reaction (PCR) and other molecular techniques may be employed for the precise identification of fungal species, especially in cases where traditional methods are not suitable.
Important Considerations
1. Sterility: Ensure that all instruments and collection materials are sterile to avoid contamination and false results.
2. Patient Instructions: Provide clear instructions to patients regarding sample collection, emphasizing the importance of obtaining material from the active border of the lesion.
3. Transportation: Transfer collected samples to the laboratory promptly, especially if cultures are needed, to maintain the viability of the fungi
KOH Preparation
A potassium hydroxide (KOH) preparation is a simple and widely used laboratory technique in the collection and processing of samples in mycology for the diagnosis of fungal infections in the skin, nails, and hair. KOH is an alkali that is used to digest keratin in samples. Keratin is a protein present in the in skin, hair, and nails. Keratin digestion allows the clear visualization of fungal elements under a microscope.
Materials Needed:
1. Specimen: Clinical specimens such as skin scrapings, hair plucking, or other material collected from the affected area.
2. 10% KOH Solution.
3. Microscope Slides and Coverslips: Clean and sterile glass slides and coverslips for mounting the specimen.
4. Microscope: A light microscope with appropriate magnification for fungal examination.
Procedure:
1. Obtain the Specimen: Place a small portion of the clinical specimen (e.g., skin scraping, hair plucking) on a clean glass slide.
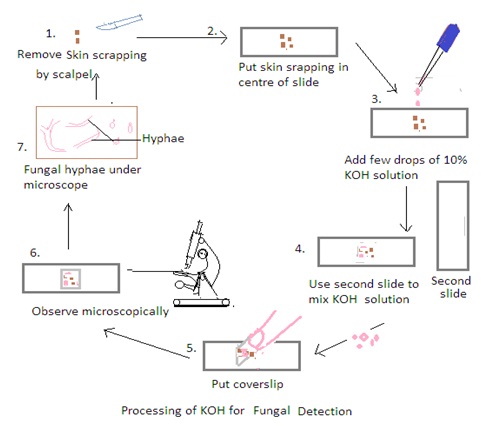
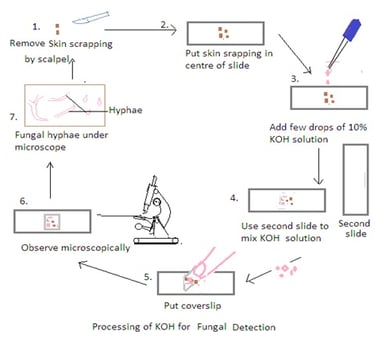
2. Add KOH to the Specimen: Apply a few drops of the 10% KOH solution to the specimen on the slide. Ensure that the specimen is well-covered by the KOH solution.
3. Mix and Disperse: Use a second clean glass slide to mix and disperse the specimen in the KOH solution. Gently press down on the specimen with the second slide to spread it out and help in the dissolution of keratin. It also ensures thorough mixing to break down the tissue and release fungal elements.
4. Cover with a Coverslip: Place a clean coverslip over the mixture to create a wet mount. Press down gently to spread the specimen uniformly and reduce air bubbles.
5. Let it Stand: Allow the KOH mount to stand for a few minutes (usually 5-10 minutes). The KOH solution dissolves keratin, making fungal elements more visible under a microscope.
6. Examine Under the Microscope: Place the prepared KOH mount on the stage of a light microscope and examine it under low and high magnifications. Fungal elements such as hyphae, spores, and other structures become more apparent after the keratin dissolution.
7. Use Staining Technique if needed: KOH preparation allows for the visualization of fungal elements. Stains such as calcofluor white or fungal-specific stains may be used to enhance visibility.
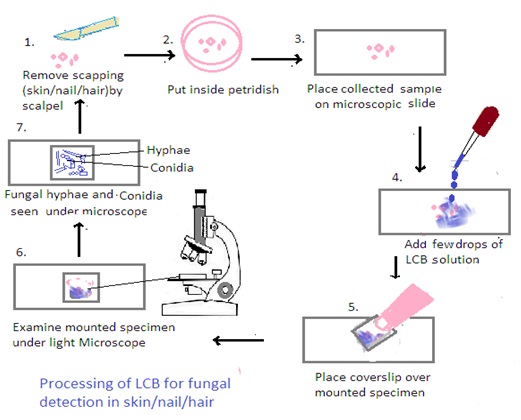
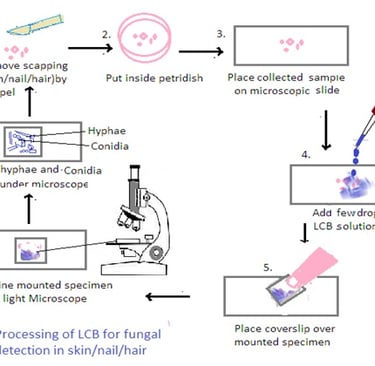
LCB (Lactophenol cotton blue)
Lactophenol cotton blue (LCB) is a mounting medium solution widely used in mycology laboratories due to its simplicity, effectiveness, and ability to provide clear and detailed microscopic images of fungal structures. It is most suitable for staining and preservation of fungal specimens. It is especially useful in the identification of various fungal structures, including hyphae, spores, and other reproductive elements. It is also used for differentiating between various fungal species and assisting in the identification of diagnostic features. The LCB solution consists of several components:
1. Lactic Acid: Lactic acid serves as a clearing agent. It helps to make the fungal structures more visible by removing cell debris and pigments. It also helps in breaking down the fungal cell walls. This makes the structures more permeable to the stain.
2. Phenol: Phenol acts as both a preservative and a fungicide. It helps to fix the fungal structures on the slide. It also prevents deterioration over time. Additionally, it inhibits the growth of fungi, bacteria, and other microorganisms that may interfere with the examination.
3. Cotton Blue: Cotton blue is a blue staining dye that imparts color to the fungal structures. It enhances their visibility under the microscope. It stains chitin, a component of fungal cell walls, and allows for the differentiation of various fungal elements.
4. Glycerol or Distilled Water: Glycerol or distilled water is often added to the solution to adjust the viscosity and aid in the spreading of the specimen on the microscope slide.
5. Storage: Store LCB in a cool, dark place to prevent deterioration.
6. Quality of the LCB Solution: The LCB solution should be fresh and well-mixed for optimal staining and visualization.
7. Sterility: Ensure that all tools, slides, and coverslips used in the process are sterile to avoid contamination
Collection of Samples
1. Skin Scrapings: Gently scrape the affected skin area with a sterile scalpel or spatula to collect scales, debris, or tissue fragments.
2. Nail Clippings: Trim a small piece of the affected nail, ensuring to include both the infected and healthy portions.
3. Hair Samples: Collect hair samples from the affected area using methods like skin scrapings or hair plucking.
Processing of Samples using Lactophenol Cotton Blue (LCB):
1. Preparation of LCB Mounting Medium: Lactophenol cotton blue (LCB) is a solution that contains lactophenol, which is a mixture of lactic acid, phenol, glycerol, and cotton blue stain. Mix the LCB solution thoroughly before use.
2. Mounting the Sample: Place a small amount of the collected material (skin scrapings, nail clippings, or hair samples) on a clean microscope slide.
3. Addition of LCB: Add a drop of LCB solution to the specimen on the slide. The LCB stain helps to clear the sample and make fungal structures more visible under the microscope.
4. Cover Slip Placement: Gently place a coverslip over the specimen, being careful to avoid trapping air bubbles. Press down gently to spread the LCB and ensure an even distribution.
5. Microscopic Examination: Examine the mounted specimen under a light microscope, using both low and high magnifications. LCB helps in visualizing fungal structures such as hyphae, conidia, and other reproductive structures.
India Ink
India ink is also known as Chinese ink. It is a traditional black ink that has been used for writing and drawing for centuries. It originated in China. The name "India ink" is a misnomer, as the ink is not actually from India; it likely got its name because it was originally traded through India.
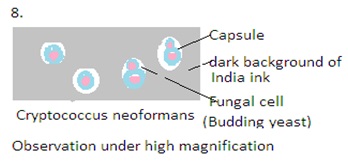
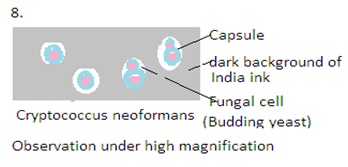
India ink is a commonly used staining technique in mycology, especially for the detection and observation of certain fungal structures, particularly Cryptococcus species. Cryptococcus is a genus of fungi that includes opportunistic pathogens known to cause cryptococcal infections, with Cryptococcus neoformans. These fungi are associated with infections such as cryptococcal meningitis, which can be particularly problematic for immunocompromised individuals.
India ink staining is highly valuable in the identification of Cryptococcus. This technique is specific to certain fungi. Other staining methods may be required for the identification of different fungal species.
Principle of India Ink Staining:
The India ink used is a suspension of carbon particles. This is dense and refracts light, creating a dark background The India ink stain is a negative staining technique. It stains the background and leaves the fungal cells unstained. Unstained fungal cells are visible against the dark background. This technique is valuable for visualizing structures that are not visible in conventional staining methods.
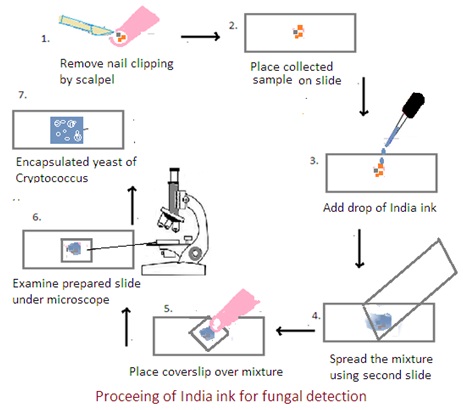
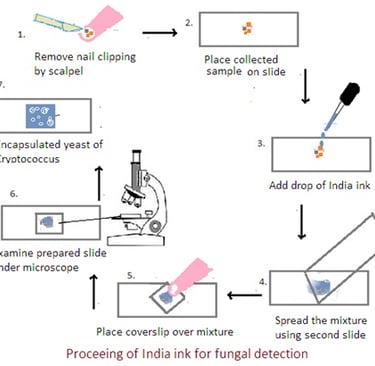
Collection of Sample:
1. Skin and Hair Scrapings: Use a blunt scalpel or the edge of a glass slide to gently scrape the affected area and collect scales, debris, or hair fragments.
2. Nail Clippings: Collect nail clippings from the affected area, ensuring that both the surface and the undersurface of the nail are included.
Procedure:
1. Preparation of Sample: Place a small amount of collected material on a glass slide.
2. Mixing with India Ink: Add a drop of India ink, covering the specimen completely. Gently mix the ink and the specimen.
3. Spreading Thin Film: Spread the mixture into a thin and even film using another clean slide. This allows for better visualization of the fungal cells against the dark background.
4. Application of Coverslip: Place a coverslip over the mixture, ensuring that the ink and specimen are spread evenly. Press down gently to minimize air bubbles.
5. Microscopic Examination: Examine the prepared slide under a microscope using a light microscope with a bright field. Use low-power objective (10X) and then transition to higher magnifications (40X and 100X). The fungal cells, particularly the encapsulated yeasts of Cryptococcus, appear as clear or unstained structures against the dark background of the India ink.
Fungal Culture media
Fungal culture media are specialized formulations used to cultivate and grow fungi in laboratory settings. These media provide the necessary nutrients, pH, and other conditions that support the growth of various fungal species. There are several types of fungal culture media, each designed to meet specific requirements for the isolation, identification, and study of fungi. The composition of these media can vary based on the nutritional needs and environmental preferences of different fungal species. The following are common fungal culture media for fungi.
Sabouraud Dextrose Agar (SDA):
It contains dextrose (glucose), peptone, and agar. It is widely used for the isolation and cultivation of yeasts and molds. The acidic pH (around 5.6) inhibits bacterial growth, allowing for the selective growth of fungi.
Applications:
1. Clinical mycology: SDA with antibiotics is commonly used in diagnostic laboratories for the isolation of pathogenic fungi from specimens like skin scrapings, nail clippings, or other clinical samples.
2. Environmental mycology: In studies where fungi from environmental samples are being isolated, the addition of antibiotics can help in obtaining pure fungal cultures without interference from bacterial contaminants.
There are two types of Sabouraud Dextrose Agar (SDA). These are Sabouraud Dextrose Agar (SDA) without antibiotics and Sabouraud Dextrose Agar (SDA) with antibiotics.
Sabouraud Dextrose Agar (SDA) without antibiotics
Composition
· Peptone: Provides a source of nitrogen and amino acids.
· Dextrose (glucose): Acts as the main carbon and energy source for fungal growth.
· Agar: Solidifying agent to create a gel-like consistency.
· pH: Typically adjusted to around 5.6, creating an acidic environment that inhibits bacterial growth but supports fungal growth.
Uses: SDA without antibiotics is a general-purpose medium widely used for the cultivation and isolation of yeasts and molds. The slightly acidic pH helps to suppress bacterial growth, making it selective for fungi. This medium supports the growth of a broad spectrum of fungal species. It is commonly employed in clinical laboratories for the isolation and identification of pathogenic fungi from clinical samples.
Sabouraud Dextrose Agar (SDA) with antibiotics:
SDA may include antibiotics such as chloramphenicol or gentamicin. Chloramphenicol inhibits the growth of bacteria by interfering with protein synthesis. Gentamicin is an antibiotic that is effective against a broad range of bacteria.
Uses: SDA with antibiotics are employed when the primary objective is to isolate fungi and eliminate bacterial contamination. In certain situations, the presence of bacteria with fungi may interfere with the isolation and identification of fungi. The addition of antibiotics helps to create a more selective medium by preventing the overgrowth of bacteria.
Cornmeal Agar (CMA):
Corn meal Agar (CMA) is a specialized fungal culture medium designed to support the growth of filamentous fungi and facilitate the observation of their morphological characteristics. It is particularly valuable in mycological research for differentiating and identifying fungal species based on their unique morphological features.
Low Nutrient Content: CMA has a lower nutrient content compared to some other fungal culture media. This makes it suitable for promoting the development of distinctive morphological structures in fungi. It is an excellent culture medium for the morphological study of the fungi.
Composition: Cornmeal agar medium contains cornmeal extract
1. Cornmeal: Provides a source of carbohydrates and nutrients.
2. Dextrose (glucose): Acts as a supplementary carbon and energy source.
3. Agar: Solidifying agent.
4. pH Indicator: Some formulations may include a pH indicator, typically bromothymol blue, which can help visualize changes in pH.
Preparation: Cornmeal agar medium contains cornmeal extract, dextrose, and agar in distilled water. Cornmeal is boiled in water to create a cornmeal extract. Cornmeal Agar is prepared by mixing cornmeal extract and dextrose in water, adjusting the pH to around 5.6, and solidifying the medium with agar.
Observation: After inoculation and incubation, characteristic features such as color, texture, and spore production, arrangement of spores are observed. This provides valuable information for identification. The medium is especially valuable for distinguishing between various genera and species of molds.
Use
1. Selective Medium for Morphological Studies
· CMA is primarily used for the cultivation and morphological characterization of filamentous fungi, especially molds.
· The composition of CMA is relatively simple, allowing for the observation of characteristic morphological features of fungi, such as conidial (asexual spore) formation and hyphal characteristics
2. Differentiation of Fungal Species
· CMA is particularly useful for differentiating fungal species based on their colony morphology and spore production
· The medium allows for the visualization of structures like conidia, conidiophores, and mycelial characteristics, aiding in the identification of fungi
Applications
1. Morphological Studies: CMA is particularly useful for observing the morphological features of filamentous fungi (molds). It allows the visualization of structures such as conidia (asexual spores), sporangiophores, and conidiophores.
2. Identification: The morphology of fungi on CMA helps in the identification of fungal species. Different species may produce characteristic patterns of growth, pigmentation, and spore formation
3. Taxonomy studies: CMA is commonly used in mycology laboratories for taxonomic studies of fungi by examining the morphology of different fungi. It supports the growth of a wide variety of filamentous fungi.
4. Research and Education: It is used in research and educational settings for teaching mycology and allowing students to observe and study the morphology of different fungi.
Brain Heart Infusion (BHI)
Brain Heart Infusion (BHI) is a nutrient-rich and versatile medium used in microbiology for the cultivation of a wide range of microorganisms. Its composition makes it suitable for supporting growth.
Features
1. Nutrient Rich: BHI is highly nutritious, supporting the growth of fastidious microorganisms. These microorganisms have more complex nutritional requirements.
2. Versatile: It is used for the cultivation and maintenance of a broad range of microorganisms. This makes it suitable for various applications in clinical, industrial, and research settings.
Composition
1. Infusion from Calf Brains and Beef Hearts: BHI is prepared using an infusion made from calf brains and beef hearts. This infusion provides a rich source of nutrients, including amino acids, vitamins, and minerals.
2. Porcine Brain Tissue: Some formulations may also include porcine (pig) brain tissue.
3. Peptones: Enzymatic digestion of protein produces peptone. Peptone provides additional amino acids and nitrogenous compounds.
4. Glucose: Acts as a carbohydrate and energy source.
5. Sodium Chloride: Provides essential ions
6. Agar (optional): BHI can be prepared as a broth or as a solid agar medium.
Preparation: BHI can be prepared as a liquid broth or as a solid agar medium depending on the specific requirements of the experiment or application.
Incubation: Inoculated BHI media are typically incubated at a temperature suitable for the growth of the microorganisms being studied.
Dr Pramila Singh
