fresh histological tissues Handling
Handling of fresh histological tissues (Frozen Section): Reception and processing of frozen tissue, Freezing microtome, and cryostat. Advantages and disadvantages of freezing microtome and cryostat. Working, care, and maintenance of freezing microtome and cryostat. Frozen section cutting, Staining - Rapid H&E - Fat stain Mounting of frozen section
Dr Pramila Singh
3/8/202412 min read
My post contentHandling of fresh histological tissues (Frozen Section): Reception and processing of frozen tissue, Freezing microtome, and cryostat. Advantages and disadvantages of freezing microtome and cryostat. Working, care, and maintenance of freezing microtome and cryostat. Frozen section cutting, Staining
- Rapid H&E,
- Fat stain.
Mounting of frozen section. Unit-II.
Handling of fresh histological tissues (Frozen Section).
Handling fresh histological tissues for frozen sections involves specific procedures to ensure optimal tissue preservation and subsequent accurate microscopic examination.
The handling of fresh histological tissues for frozen sections involves rapid processing, careful embedding, cryosectioning, quick staining, immediate microscopic examination, diagnostic decision-making, tissue preservation, communication with the surgical team, documentation, and quality control measures to ensure accurate and timely results.
Reception and processing of frozen tissue
The reception and processing of frozen tissue in a histological laboratory involve several steps. These steps ensure the preservation of cellular structures and the generation of high-quality tissue sections. The following are the main aspects of the reception and processing of frozen tissue./.
1. Tissue Reception:
Objective: Receive the frozen tissue specimen for histological analysis.
Procedure: Verify the documents, including patient information, sample source, and any relevant clinical details. Ensure proper storage, handling, and care to prevent thawing during transport.
2. Tissue Identification and Labeling:
Objective: Maintain accurate sample identification throughout the process.
Procedure: Label the specimen container with unique identifiers, cross-referencing it with patient details. Confirm that the labeled information matches the accompanying documentation.
3. Fixation:
Objective: Prevent deterioration of tissue samples during storage and transportation. Hardening of soft tissue. Convert of semifluid consistency of cells/tissues to semisolid.
Procedure: Use 10% formaldehyde solution (formalin) as a fixative. Ensure the thickness of the tissue is not more than 3 mm in thickness.
Freezing: Laboratory ingratiation requires rapid diagnosis. Freezing prevents the deterioration of tissue samples. It is useful for the demonstration of components soluble in alcohol or clearing agents.
Procedure: Place a drop of water in the pre-cooled block holder. Place the tissue sample in a drop of water. Place the block holder in a bath of alcohol or acetone containing dry ice for the sample or expose the tissue to carbon dioxide gas for rapid freezing.
4. Thawing of Tissue:
Objective: Bring the frozen tissue to a manageable temperature for further processing.
Procedure: Thaw the tissue specimen appropriately. Take care to avoid rapid or uneven thawing that could affect tissue integrity.
5. Embedding in Optimal Cutting Temperature (OCT) Compound:
Objective: Provide support for cryosectioning and maintain tissue structure.
Procedure: Embed the thawed tissue in the Optimal Cutting Temperature (OCT) compound. Ensure complete coverage. This step facilitates the creation of tissue blocks for cryosectioning.
6. Cryosectioning:
Objective: Obtain thin and consistent tissue sections for microscopic examination.
Procedure: Use a cryostat to cut thin sections from the OCT-embedded tissue blocks. The sections are typically around 5 to 10 micrometers thick. Cut 6 to 8 sections.
7. Mounting on Slides:
Objective: Transfer tissue sections onto glass slides for further processing.
Procedure: Float tissue sections in distilled water. Collect the cut sections on glass slides, ensuring proper orientation. Use absolute alcohol to cover the cut section. Rapidly transfer to a Petri dish containing 0.5 to 1% celloidin. Keep it for 5 to 10 seconds. Drain excess celloidin and allow the cut section for 5 to 10 seconds. This step prepares the tissue for subsequent staining and analysis.
8. Staining Techniques:
Objective: Highlight specific structures for microscopic examination.
Procedure: Apply quick staining methods, such as hematoxylin and eosin (H&E), to provide contrast and reveal cellular details. Additional special stains may be used for specific diagnostic purposes.
9. Microscopic Examination:
Objective: Analyze stained tissue sections for diagnostic evaluation.
Procedure: Examine the stained sections under a microscope, assessing cellular morphology and making diagnostic observations.
Freezing microtome
A freezing microtome is a specialized laboratory instrument used to cut thin slices (sections) of frozen biological specimens for microscopic examination. The freezing microtome allows researchers and pathologists to obtain thin, precise sections of tissues without the need for chemical fixation and paraffin embedding.
Principle: The freezing microtome operates on the principle of cutting frozen tissues. Tissue samples (Specimens) are rapidly frozen to a temperature to make tissues hard and suitable for section cutting without distortion.
Components:
1. Microtome Knife: The microtome is equipped with a sharp blade or knife. A sharp blade or knife is used to cut thin sections of the frozen tissue. The knife can be adjusted for thickness to obtain slices of varying thicknesses.
2. Specimen Holder: The frozen tissue specimen is mounted on a specimen holder. The specimen holder can be adjusted to cut the tissue of the required thickness
Applications:
1. Histology and Pathology: Freezing microtomes are commonly used in histological and pathological studies to prepare fresh-frozen tissue sections. for diagnostic and research purposes.
2. Neuroscience: Researchers in neuroscience use freezing microtomes to obtain thin brain sections for studies on brain anatomy and pathology.
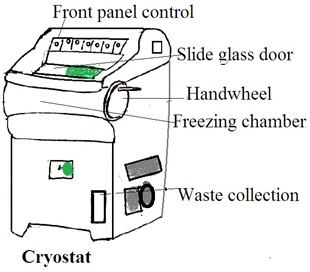
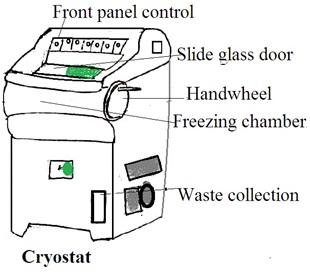
Cryostat
A cryostat is a device or system designed to maintain extremely low temperatures, near absolute zero (0 Kelvin or -273.15 degrees Celsius). This low temperature induces specific physical or chemical behaviors in materials. Or, this low temperature is low enough for the operation of specialized equipment.
It is used in various scientific and industrial applications where very low temperatures are required.
The Components of a Cryostat
1. Cryogenic Vessel (Dewar flask): This is a container that holds cryogenic fluids, such as liquid nitrogen or liquid helium. The dewar flask is a well-insulated double-walled vessel to minimize heat transfer and maintain low temperatures.
2. Thermal insulation: Cryostats are equipped with advanced thermal insulation. Common insulation materials include vacuum insulation panels, multi-layered reflective shields, and other high-efficiency insulating materials.
3. Temperature Control System: A temperature control system is a refrigeration unit. It is used to cool the interior of the cryostat to extremely low temperatures. It uses liquid nitrogen, liquid helium, or closed-cycle refrigerators.
4. Cryogenic Fluid Circulation System: In some cryostats, there is a system for circulating and managing the flow of cryogenic fluids to maintain a constant low temperature.
5. Sample Holder: The sample holder or stage is to hold the sample for cooling.
6. Vacuum Pump: The cryostat may have a vacuum pump to maintain a low-pressure environment in the insulating space. It reduces heat transfer through conduction
The difference between Freezing Microtome and Cryostat
The terms "cryostat" and "freezing microtome" lead to some confusion. But both are different instruments. In many cases, a cryostat and a freezing microtome work together such as in the preparation of frozen tissue sections.
Cryostat: A cryostat is a device that maintains a low-temperature chamber. It is equipped with a refrigeration system to maintain very low temperatures.
Freezing Microtome: A freezing microtome is a cutting instrument. It is used for sectioning thin slices of biological specimens for microscopic analysis.
Cryostats: In a broader sense, the term "cryostat" may be used to describe a combined system that includes both the low-temperature chamber and a microtome for cutting frozen sections.
Freezing microtome: The freezing microtome is specifically focused on the mechanical process of cutting frozen tissues into thin sections
Cryostats are used for various applications, including the preservation of tissues in their natural state without the need for fixation and paraffin embedding.
Freezing Microtome: A freezing microtome is designed to work with pre-frozen tissues. It may not have a built-in refrigeration system but relies on the pre-frozen state of the tissue, often prepared using a separate freezing apparatus.
Advantages and disadvantages of freezing microtome and cryostat
A microtome is a laboratory instrument used to cut extremely thin slices of biological specimens for microscopic examination. Freezing microtomes operate at low temperatures and are commonly used for cutting frozen tissues. The following are the advantages and disadvantages of freezing microtome.
Advantages
1. Preservation of Tissue Structure: Freezing microtomes allow the cutting of tissues below freezing temperatures. This preserves the natural structure of the tissues. This is useful for delicate structures that may be damaged during paraffin embedding.
2. Rapid Processing: Frozen sections can be prepared quickly compared to other methods. This allows for rapid analysis of tissues. This is useful where a quick diagnosis or examination is required, such as in surgical pathology.
3. No chemical processing: Freezing microtomes do not require chemicals. This can be advantageous for studying molecules or structures that may be affected by chemical treatments.
4. Suitable for Soft Tissue: Freezing microtomes are well-suited for cutting soft tissues.
5. Minimize Artifacts: The process of freezing and cutting allows for minimal distortion and artifact formation. This leads to a more accurate representation of tissue structures.
Disadvantages:
1. Limited Tissue Types: Not all tissues freeze well. Certain tissues may not be suitable for sectioning using a freezing microtome.
2. Ice Crystal Formation: Ice crystals can form within the tissue during the freezing process. Ice crystals may cause damage to cellular structures. Care must be taken to minimize ice crystal formation. Cryoprotectants are used to prevent ice crystal formation.
3. Variable Section Quality: Achieving constant and high-quality sections can be challenging with freezing microtomes. The thickness and quality of sections may vary. It requires skill and experience to obtain reliable results.
4. Equipment and Maintenance: Cryostats are complex instruments that require maintenance and careful calibration. The cost of equipment and ongoing maintenance are relatively high.
5. Limited Section Thickness: The thickness of sections that can be obtained with freezing microtomes is often limited compared to paraffin sections. This limitation may affect certain types of analyses or studies.
Working, care, maintenance of microtome and cryostat
A freezing microtome is a specialized piece of laboratory equipment used for cutting thin sections of frozen specimens.
Working Principle:
1. Freezing and Sectioning:
The cryostat has a freezing chamber for the specimen. The specimen is placed in the freezing chamber and frozen to the desired temperature.
The frozen specimen is then transferred to the cutting chamber.
A microtome mechanism allows controlled slicing of the frozen specimen into thin sections.
Sections are mounted on slides for further analysis.
2. Temperature Control: Precise temperature control is critical. The cryostat maintains a low temperature, even below freezing. It keeps the specimen frozen during the cutting process.
3. Microtome Mechanism: The microtome component allows for controlled advancement of the specimen, ensuring uniform section thickness.
Care and Maintenance
1. Cleaning:
Regularly clean the microtome and cryostat to prevent contamination. Proper cleaning ensures accurate results.
Use appropriate cleaning agents and methods to avoid damage to sensitive components.
2. Specimen Holder: Clean and sterilize the specimen holder regularly to prevent cross-contamination between specimens.
3. Knife Blade: Keep the knife blade sharp and in good condition.
Regularly clean and check for any signs of wear. Replace the blade as needed to maintain optimal cutting performance.
4. Temperature Control:
Regularly calibrate and monitor the temperature settings to ensure accurate freezing.
Clean and check the refrigeration system for any issues that may affect temperature stability.
5. Vacuum System: If the cryostat has a vacuum system, ensure it is functioning properly. A vacuum removes moisture during freezing and improves section quality.
6. Lubrication: Lubricate moving parts according to the manufacturer's recommendations. It prevents friction and ensures smooth operation.
7. Alignment and Calibration: Regularly check and calibrate the microtome mechanism for precise section thickness.
Verify the alignment of the cutting blade to ensure accurate and consistent sections.
8. Cryoprotectants: Cryoprotectants minimize ice crystal formation and improve the quality of frozen sections.
Frozen section cutting:
Frozen section cutting is a rapid diagnostic technique used in pathology. This method allows for the quick examination of tissue samples. The following steps are followed in the frozen section cutting process:
1. Sample Collection:
A small piece of tissue is removed during surgery for analysis. The tissue sample should be representative of the lesion or area of interest.
2. Tissue Embedding: The tissue is embedded in a special compound called OCT (optimal cutting temperature) compound or other freezing mediums. OCT helps to preserve the tissue's cellular structure.
3. Freezing: The embedded tissue is rapidly frozen using a cryostat or a freezing microtome. A cryostat is a device that maintains extremely low temperatures. Cryostat allows precise sectioning of frozen tissues.
4. Sectioning: The frozen tissue block is then sectioned into thin slices (usually 5 to 10 micrometers thick) using a microtome within the cryostat. The sections are collected on glass slides.
5. Staining: The frozen tissue sections are quickly stained with special dyes. It highlights cellular structures and details. Common stains used include hematoxylin and eosin (H&E), which provide contrast between the cell nuclei (hematoxylin) and the cytoplasm (eosin).
6. Examination: The stained sections are examined under a microscope by a pathologist. The pathologist looks for characteristic features that can help in making a preliminary diagnosis.
7. Reporting: Based on the findings, the pathologist provides a report.
Frozen section cutting is particularly useful in surgeries where immediate information about the nature of a tissue lesion is critical. For example, it may be employed during cancer surgeries. The surgeon determines based on a report about the need to remove additional tissue, perform further procedures, or if the current resection is sufficient.
It's important to note that frozen section analysis provides quick results. However, the final and more detailed pathology report is obtained through traditional formalin-fixed, paraffin-embedded tissue processing. But this process takes more time but provides a more comprehensive analysis.
Staining - Rapid H&E and Fat stain
Rapid H&E Stain:
Hematoxylin and eosin (H&E) staining is a widely used histological staining method that provides contrast between different structures within tissues.
The staining process involves two main components: hematoxylin and eosin. Hematoxylin stains cell nuclei blue, and eosin stains cytoplasm and extracellular structures pink. The conventional H&E staining process usually takes several hours. However, if a rapid staining is essential then a modified or rapid H&E staining process is used.
Preparation of the Hematoxylin and eosin (H&E) stain
1. Hematoxylin solution
· Hematoxylin 1 gm
· Absolute Alcohol 10mL
· Aluminium Potassium Sulphate 20 gm
· Distilled Water 200 mL
· Mercuric Oxide 0.5 gm
2. Eosin Aqueous 5% in water.
The following steps are followed for a rapid H&E stain.
1. Fixation: The tissue is initially fixed using a fixative solution (such as hot 10% formal saline) to preserve its structure. The thickness of tissue to be sectioned should not be more than 3 mm.
2. Freezing: Freeze the tissue with a burst of carbon dioxide.
3. Section cutting: Cut 6 to 8 tissue sections. Float the tissue section in distilled water and collect the tissue section on the slide.
4. Hardening:
Cover the tissue section on the slide with alcohol.
Rapidly transfer tissue section to Petri-dish containing 0.5% to 1% celloidin for 5 to 10 seconds. Drain out excess celloidin solution.
Allow the tissue section to dry.
Cover the tissue section with 70% alcohol for 10 to 20 seconds. It makes the tissue section hard.
7. Staining:
· Deep the tissue section quickly in hematoxylin for 1 minute. It stains the nuclei.
· Wash it with distilled water.
· Counter-stain it with aqueous eosin. It stains the cytoplasm.
· Wash the stain with distilled water.
8. Dehydration: Rinse with 95% alcohol and then dehydrate it using absolute alcohol.
9. Clearing: Clear it with xylene.
10. Mounting: Mount in DPX (Dibutylphthalate Polystyrene Xylene).
The rapid H&E stain provides a quick overview of tissue morphology. However, it may not be as detailed or permanent as the standard H&E stain. This method is often used if immediate results are required, such as during frozen section analysis in surgery
Difference between Rapid Hematoxylin and eosin (H&E) staining and Hematoxylin and eosin (H&E) staining.
Rapid Hematoxylin and Eosin (H&E) staining is faster than the standard H&E staining process. The following are the reasons to make rapid H&E staining acts faster:
1. Reduced Fixation Time: In rapid H&E staining, the fixation step is minimized. This step involves preserving tissue structure using a fixative solution, typically formalin. Standard H&E staining may involve longer fixation times to ensure optimal tissue preservation.
2. Shortened Dehydration and Clearing Steps: In rapid H&E staining, the dehydration and clearing steps are shortened compared to the standard H&E staining.
3. Accelerated Staining: The staining steps with hematoxylin and eosin are carried out more rapidly in the rapid H&E staining protocol. The tissue spends less time in each staining solution compared to the standard protocol.
4. Applicability to Frozen Sections: Rapid H&E staining is particularly suitable for frozen sections obtained during surgery.
Fat Stain:
Staining for fat is a specific technique used to highlight fat droplets within tissues. One common stain for detecting fat is the Oil Red O stain. The following are the steps of the process:
1. Fixation: The tissue is fixed using a fixative solution to preserve its structure.
2. Sectioning: The tissue is cut into thin sections using a microtome.
3. Staining with Oil Red O: The tissue sections are treated with a solution of Oil Red O dye, which selectively stains lipids and fat droplets. The sections may be counterstained with hematoxylin to provide contrast.
4. Mounting: After staining, the sections are mounted on glass slides and coverslipped for microscopic examination.
Under the microscope, fat droplets stained with Oil Red O appear red, while other cellular components may be counterstained with hematoxylin to provide additional contrast.
This fat staining technique is commonly used in histology and pathology to identify the presence and distribution of fat within tissues. It is especially useful in assessing conditions such as fatty liver disease or in the examination of adipose tissue.
Mounting of frozen section.
Mounting frozen sections is a step in the preparation of tissue samples for microscopic examination during frozen section analysis. This process involves placing the frozen tissue sections onto glass slides, allowing them to adhere to the slide's surface for staining and microscopic observation. The following are steps of the process of mounting frozen sections:
1. Sectioning: Before mounting, tissue samples are frozen using a cryostat or similar equipment. The cryostat allows for the precise sectioning of frozen tissues into thin slices (typically around 5 to 10 micrometers thick). The frozen tissue blocks are mounted on the microtome within the cryostat.
2. Tissue Transfer: The frozen tissue sections are carefully transferred from the cryostat onto glass slides. The slides are usually pre-cooled to enhance the adhesion of the tissue sections.
3. Mounting Medium: A mounting medium is applied to the surface of the glass slide. The mounting medium secures the tissue sections in place and protects the morphology of the tissue in further steps. The mounting medium contains glycerol or a synthetic resin.
4. Flattening and Spreading: Place a cover slip gently on the tissue sections present on a glass slide. The cover slip flattens and spreads the tissue on the glass slide. This helps minimize distortion and ensures even contact between the tissue and the slide.
5. Removal of Excess Medium: Wiped away excess mounting medium carefully present around the edges of the coverslip.
6. Quick Freezing: Quick freezing step secure mounted tissue sections.
7. Storage or Immediate Staining: The mounted slides may be stored for a short period or immediately processed for staining. For frozen section analysis, rapid stains such as hematoxylin and eosin (H&E) are commonly used to provide quick diagnostic information during surgery.
8. Microscopic Examination: Once the mounting medium has been set and the tissue sections are firmly attached to the slide, the slides are ready for microscopic examination.
Dr Pramila Singh
