Electrophoresis
Electrophoresis: Principle and Theory of Electrophoresis, Types of Electrophoresis, Clinical Significance/Applications of Electrophoresis. HSBTE IVth Semester. Biochemistry.
BIOCHEMISTRY
4/12/20247 min read
Electrophoresis: Principle and Theory of Electrophoresis, Types of Electrophoresis, Clinical Significance/Applications of Electrophoresis. HSBTE. IVth Semester. Biochemistry.
Electrophoresis
“Electrophoresis is a laboratory technique that separates charged particles based on their mobility in an electric field”.
Principle and Theory of Electrophoresis
Principle of Electrophoresis: Electrophoresis is based on the movement of charged particles in an electric field. An electric field is applied across a medium (such as a gel or a capillary tube) containing charged particles. Charged particles will migrate toward the electrode of the opposite charge. The rate of migration is influenced by the charge of the particles, the size of the particles, the strength of the electric field, and the properties of the medium.
Theory of Electrophoresis: Electrophoresis involves the forces acting on charged particles in an electric field. These forces determine migration patterns. The following are the theoretical aspects of electrophoresis:
1. Electrophoretic Mobility:
· Electrophoretic mobility (μ) is the rate at which a charged particle migrates in an electric field.
· It is calculated by the equation: μ=v/E. v is the particle's velocity and E is the electric field strength.
· Mobility is influenced by the charge and size of the particle.
2. Separation Based on Charge and Sizen
· Charged particles experience a force (F) in an electric field. Coulomb's law calculates force in an electric field. F=qE, Where q is the charge on the particle and E is the electric field strength.
· Smaller particles with higher charge migrate faster and separate.
3. Medium Influence: The gel or capillary medium provides a sieving effect. This influences the immigration of particles based on their size.
In gel electrophoresis, smaller particles move more easily through the pores of the gel. This results in differential immigration and separation.
4. Visualization and Detection: After electrophoresis, the separated particles need to be visualized and detected. Common methods include staining with dyes, fluorescent tags, or autoradiography for nucleic acids.
5. Applications: Electrophoresis is widely used in molecular biology for DNA, RNA, and protein analysis. It plays a crucial role in techniques like agarose gel electrophoresis for DNA separation, SDS-PAGE for protein separation, and capillary electrophoresis for high-resolution separations.
The mobile phase in electrophoresis
The mobile phase is a medium for the movement of the charged particles and their separation based on their charge-to-mass ratio. The liquid or gel matrix is used as a mobile phase in the electrophoresis. The composition of the mobile phase varies. It depends on the type of electrophoresis. A buffer solution is a mobile phase in gel electrophoresis (e.g., agarose gel electrophoresis or polyacrylamide gel electrophoresis). A buffer solution maintains a stable pH environment. The mobile phase in capillary electrophoresis (CE) is an electrolyte solution present within the capillary tube.
Support Media in electrophoresis
Separation of particles in the sample by electrophoresis requires support media. Paper, Agar and Agarose, cellulose acetate, polyacrylamide, etc are used as support media.
Factors affecting Electrophoresis
1. Charges: Particles carrying more charges migrate to longer distances.
2. Molecule Size: Smaller particles carrying more charge move faster than larger particles carrying less charge.
3. Voltage: An increase in voltage increases the rate of migration of charged particles.
4. Distance between electrodes: An increase in distance between electrodes decreases the rate of migration of charged particles.
5. pH: The charged particle's migration direction depends upon the pH of the electrophoresis media.
6. Electrophoresis media ionic strength: Increase in ionic strength of electrophoresis media decreases the migration rate of charged particles.
Types of Electrophoresis
Electrophoresis is a laboratory technique used for the separation of charged particles based on their mobility in an electric field. There are several types of electrophoresis. The following are some common types of electrophoresis:
Gel Electrophoresis: It is of the following types:
i.Agarose Gel Electrophoresis: It is used for the separation of nucleic acids, such as DNA and RNA.
Principle: Agarose is a polysaccharide derived from seaweed. It forms a gel matrix through which DNA molecules migrate. Smaller DNA fragments move faster. The separation is based on size.
ii. Polyacrylamide Gel Electrophoresis (PAGE):
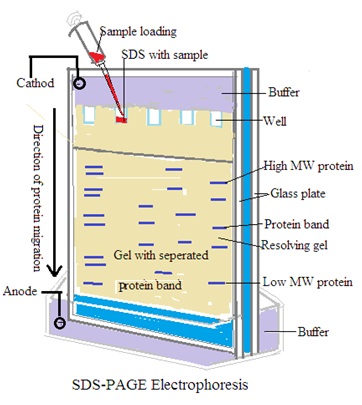
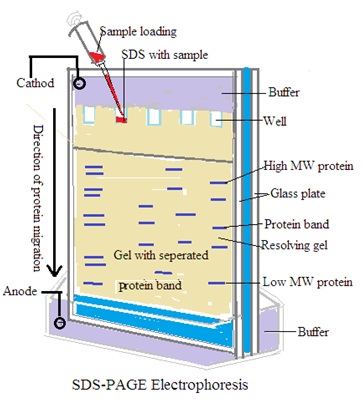
SDS-PAGE: sodium dodecyl sulfate (SDS) is used to denature proteins. Separation of proteins based on size after denatured proteins.
Native PAGE: It involves the separation of proteins under native conditions without denaturation.
Principle: Polyacrylamide gels provide higher resolution for smaller molecules like proteins. SDS-PAGE separates proteins based on size, while native PAGE separates them based on size and charge.
iii. Pulse-Field Gel Electrophoresis (PFGE): It is used for separating large DNA fragments, such as genomic DNA.
Principle: PFGE employs alternating electric field pulses. These electric pulses cause large DNA fragments to reorient and move through the gel. This technique is useful for DNA fingerprinting and chromosome analysis.
iv. Two-Dimensional Gel Electrophoresis (2D-GE): Separation of complex mixtures of proteins for proteomic analysis.
Principle: It combines two separation dimensions: isoelectric focusing in one direction and SDS-PAGE in the other. It provides higher resolution and allows the identification of individual proteins in a mixture.
v. Cellulose Electrophoresis:
Capillary Electrophoresis (CE): It is used for high-resolution separation of charged molecules such as DNA, RNA, proteins, and small molecules.
Principle: Analytes migrate through a narrow capillary filled with an electrolyte under the influence of an electric field. Capillary electrophoresis (CE) offers efficient separation and is often used in DNA sequencing.
Isoelectric Focusing (IEF): Separation of proteins based on their isoelectric points (pI).
Principle: Proteins migrate to the pH at which their net charge is zero. This is their isoelectric point. The separation is achieved in a gradient pH gel.
Affinity Electrophoresis (Immunoelectrophoresis): It is used to study interactions between molecules, such as antigen-antibody binding.
Principle: Add ligands with an affinity for specific molecules into the gel. Molecules of interest bind to these ligands. This bonding alters their electrophoretic mobility.
It detects and quantifies proteins based on their interactions such as antigen-antibody interaction.
Gel Electrophoresis
Gel electrophoresis is a laboratory technique used to separate and analyze macromolecules based on their size, charge, etc. Examples of macromolecules are such as DNA, RNA, and proteins.
Principle of Gel Electrophoresis:
The principle of gel electrophoresis is based on the movement of charged molecules through a gel matrix under the influence of an electric field. Gel electrophoresis separates bio-molecules such as DNA, RNA, and proteins based on their size, charge, or structure. The following are breakdown of the principle of gel electrophoresis:
1. Gel Matrix: The gel matrix provides a porous network through which molecules can migrate.
2. Electric Field: An electric field is applied across the gel matrix by connecting electrodes to a power supply. Electrodes create a positive pole (anode) and a negative pole (cathode). The charged molecules within the sample move through the gel toward the oppositely charged electrode.
3. Migration of Charged Molecules: The rate of migration depends on the size and charge of the molecules. Smaller molecules move more quickly through the gel matrix than larger ones. Additionally, molecules with a higher net charge move more rapidly than those with lower net charges.
4. Separation Based on Size and Charge: molecules become separated based on molecules size and charge. Smaller molecules travel farther through the gel matrix than larger ones. This leads to the separation of molecules based on size. Additionally, molecules with similar sizes are separated based on differences in charge. More highly charged molecules migrate faster than less charged ones.
5. Visualization and Analysis: The separated molecules are visualized using stains or dyes that bind to the molecules. For example, ethidium bromide is commonly used to stain DNA molecules. Coomassie Brilliant Blue is used to stain proteins.
The procedures of two commonly used gel electrophoresis:
A. Agarose Gel Electrophoresis for Nucleic Acids:
Preparation of Gel: Dissolving agarose powder in an appropriate buffer solution. (e.g., Tris-acetate-EDTA, TAE, or Tris-borate-EDTA, TBE). Heat the mixture until the agarose is completely dissolved. Allow it to cool slightly. Pour agarose solution into a gel mold. Insert a comb into the gel mold to create wells for loading samples.
Sample Preparation: Mix the nucleic acid samples with loading dye. Heat the samples briefly (usually at 65-70°C) to denature any secondary structures.
Electrophoresis: Submerge the gel in an electrophoresis buffer (e.g., TAE or TBE) in an electrophoretic chamber. Load the samples into the wells using a micropipette. Apply an electric field across the gel by connecting the electrodes to a power supply. Allow the samples to migrate through the gel for a specific period, typically at constant voltage (e.g., 100 V). Monitor the migration progress until the desired separation is achieved.
Visualization and Analysis: After electrophoresis, remove the gel from the chamber and visualize the separated nucleic acids under UV light. Stain the gel with a nucleic acid stain (e.g., ethidium bromide) and photograph the gel using a gel documentation system. Analyze the gel to determine the sizes of the nucleic acid fragments based on their migration distances.
B. Polyacrylamide Gel Electrophoresis (PAGE) for Proteins:
Gel Preparation: Prepare a polyacrylamide gel by mixing acrylamide and bis-acrylamide monomers. Add a suitable buffer and a free-radical initiator. Then pour the mixture between glass plates and insert a comb to create wells.
Sample Preparation: Denature the protein samples by heating them in the presence of a denaturing agent (e.g., sodium dodecyl sulfate, SDS) and a reducing agent (e.g., dithiothreitol, DTT). Mix the denatured samples with a loading buffer. Buffer contains tracking dyes for visualization.
Electrophoresis: Submerge the gel in a buffer (e.g., Tris-glycine or Tris-tricine) in an electrophoretic chamber. Load the samples into the wells using a micropipette. Apply an electric field across the gel by connecting the electrodes to a power supply. Allow the samples to migrate through the gel for a specific period. at constant voltage (e.g., 100 V). Monitor the migration progress until the desired separation is achieved.
Visualization and Analysis: Remove the gel from the chamber after electrophoresis. Visualize the separated proteins using staining techniques (e.g., Coomassie Brilliant Blue) or immunoblotting. Photograph the gel using a gel documentation system.
Analyze the gel to determine the sizes of the protein bands.
Clinical significance/applications of Electrophoresis
The following are the clinical Significance of Electrophoresis
Quality Control: Electrophoresis is used in clinical laboratories for quality control of various biological products. Electrophoresis ensures accurate and reliable results in diagnostic testing.
Protein Analysis: It is used to separate and analyze proteins. It helps in the diagnosis of conditions such as multiple myeloma or liver diseases where abnormal protein patterns may be present.
Hemoglobin Electrophoresis: Electrophoresis is essential to identify various haemoglobin variants. It helps to diagnose disorders like sickle cell anemia and thalassemia.
DNA Fragmentation: Electrophoresis is used in molecular diagnosis to separate and visualize DNA Fragments. It is used in DNA Fingerprinting, paternating testing, and genetic disease analysis.
Enzyme Analysis: Electrophoresis is used to study enzyme activity to identify specific enzyme deficiencies. This contributes to diagnosing metabolic disorders.
Serum and Urine Analysis: Electrophoresis of these body fluids helps to identify abnormalities like the detection of monoclonal gammography in serum or protein urea in urine.
Drug Monitoring: Electrophoresis is used to asses drug concentrations and study drug-protein interactions.
Genetic Research: Electrophoresis helps scientists in genetic research. It helps scientists to understand DNA, RNA, and protein structures.
Dr Pramila Singh
