Cytological Special Stains
Cytological Special Stains: Principle, Technique & Interpretation of PAS (Periodic Acid Schiffs reagent Stain). Principle, Technique & Interpretation of Zeihl Neelson’s (ZN) Stain (AFB). HSBTE DMLT Unit V. Immunopathology and Cytology
HISTOPATHOLOGY
Dr Prmila Singh
4/13/20243 min read
Cytological Special Stains: Principle, Technique & Interpretation of PAS (Periodic Acid Schiffs reagent Stain). Principle, Technique & Interpretation of Zeihl Neelson’s (ZN) Stain (AFB). HSBTE DMLT Unit V. Immunopathology and Cytology.
Principle, Technique & Interpretation of PAS (Periodic Acid Schiffs reagent Stain)
Principle:
The PAS stain is a histochemical technique used in histology and pathology to detect the presence of carbohydrates, specifically glycogen and mucosubstances in tissues.
The PAS reaction involves two main steps: oxidation and Schiff's reagent reaction.
Periodic acid (PAS) oxidizes the diol functional groups of carbohydrates, breaking them into aldehyde groups.
Schiff's reagent is a fuchsin-based solution It reacts with the aldehyde groups to form a magenta-colored complex.
The magenta coloration indicates the presence of glycogen or mucosubstances, helping highlight specific cellular structures or pathologic changes.
Technique
Tissue Preparation: Tissues are fixed, embedded in paraffin, and sectioned into thin slices.
Deparaffinization: Paraffin is removed using xylene or similar solvents from the tissue sections.
Periodic Acid Treatment: Tissue sections are treated with periodic acid. This step oxidizes the carbohydrates.
Rinse: Sections are rinsed to remove excess periodic acid.
Schiff's Reagent: Schiff's reagent is applied to the tissue sections. It reacts with the oxidized carbohydrates and forms a colored complex.
Counterstaining: Hematoxylin or other counterstains may be used to provide contrast to the PAS-stained structures.
Dehydration and Mounting: The sections are dehydrated, cleared, and mounted for microscopic examination.
Interpretation
Glycogen Staining: PAS stain is particularly useful for highlighting glycogen within cells. Glycogen is a storage form of glucose found in cells. A magenta color indicates its presence.
Mucosubstances: PAS staining also detects mucosubstances. Mucosubstances are glycoproteins containing carbohydrates. These substances are found in mucus-secreting cells and can be stained magenta with PAS.
Cellular Structures: PAS staining is commonly used to highlight specific cellular structures, such as the basement membrane in epithelial tissues, goblet cells in the gastrointestinal tract, and fungal elements in tissues.
Pathological Changes: PAS staining reveals pathological changes in tissues, such as abnormal glycogen accumulation (glycogen storage diseases) or abnormal mucin production (e.g., in mucinous tumors).
Differential Diagnosis: The PAS stain can aid in the differential diagnosis of various conditions, such as liver diseases, tumors, and certain infectious diseases.
Tips
Controls: Positive and negative controls should be included in each staining batch to ensure the reliability of results.
Microscopic Examination: Interpretation is done under a microscope. The intensity and pattern of staining provide valuable information about the nature of the tissues.
Principle, Technique & Interpretation of Zeihl Neelson’s (ZN) Stain (AFB).
Principle of Ziehl-Neelson’s Stain
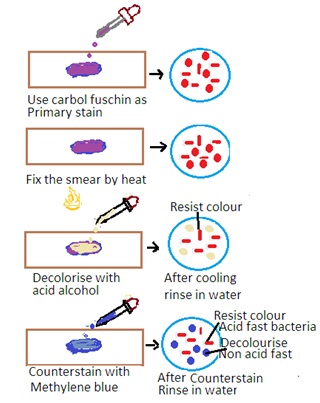
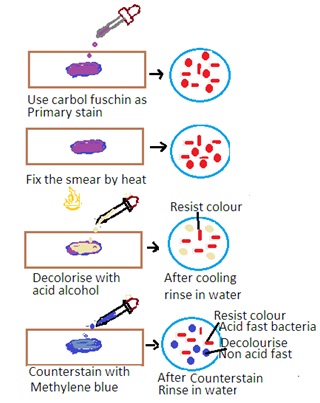
The Ziehl-Neelsen stain is a special staining technique used for the detection of acid-fast bacteria (AFB) such as Mycobacterium tuberculosis (the causative agent of tuberculosis) and Mycobacterium leprae (the causative agent of leprosy). The stain is based on the property of mycobacteria to resist decolorization by acid-alcohol after staining with carbol fuchsin. The principle has the following steps
Primary Stain: The specimen is initially stained with carbol fuchsin. Carbon fuscin is a red dye containing phenol. This dye penetrates the mycobacterial cell wall, and the cells retain the stain due to their high lipid content.
Heat fixation: The stained specimen is heated to enhance the penetration of the dye into the mycobacterial cells.
Decolorization: Acid-alcohol is used as a decolorizing agent. Acid-fast bacteria resist decolorization due to the lipid-rich cell wall. Other bacteria are decolorized.
Counter Staining: The smear is counterstained with a contrasting color, usually methylene blue, to visualize non-acid-fast bacteria.
Additional Points for Mycobacterium leprae:
Modified Zeihl-Neelsen Stain for leprae: A modified Ziehl-Neelsen stain is used for the detection of Mycobacterium leprae. This modification is required due to the low number of bacilli in leprosy lesions. A prolonged staining and careful examination is carried out..
Significance of Ziehl-Neelsen Stain:
Specific for Acid-Fast Bacteria: The Ziehl-Neelsen stain is highly specific for acid-fast bacteria, making it a crucial tool for the diagnosis of tuberculosis and leprosy.
Rapid Detection: The stain is relatively quick and can provide rapid results, facilitating timely diagnosis and treatment initiation.
Field Use: Ziehl-Neelsen stain is used in resource-limited settings. It is very useful in the field due to its simplicity and effectiveness.
Interpretation of Zeihl-Neelsen Stain
Acid-Fast Bacteria: Acid-fast bacteria will appear as bright red or pink rods under the microscope. It indicates that they have retained the carbol fuchsin stain.
Non-Acid Fast Bacteria: Other bacteria and cellular components will appear blue or green due to the counterstaining with methylene blue.
Clinical Correlation: The presence of acid-fast bacilli in clinical specimens is significant and requires correlation with clinical and other laboratory findings for a definitive diagnosis.
