Automation in Biochemistry
Automation in Biochemistry: Definition and introduction of auto analyzers in clinical biochemistry. Classification and types of Auto analyzers. ANALYTICAL CLINICAL BIOCHEMISTRY, IVth Semester. HSBTE DMLT. Unit V.
Dr Pramila Singh
5/5/20244 min read
Automation in Biochemistry
Automation is the use of automated systems and technologies to perform various laboratory experiments. Biochemistry deals with the study of the biochemicals and chemical processes that occur within living organisms. These instruments automate the process of sample handling, mixing, reaction, and analysis. This increases the speed and accuracy of testing and reduces the need for manual intervention. Automation improves efficiency, accuracy, and output.
Definition and introduction of auto analyzers in clinical biochemistry
“Auto analyzer is automated systems designed for the analysis of clinical samples in the field of the clinical laboratory”. These instruments are used in medical laboratories to perform a wide range of biochemical tests on blood, urine, and other body fluids.
“Auto analyzers automate the process of sample handling, mixing reagents, and analyzing results”. This reduces the need for manual involvement and increases the efficiency of clinical laboratory workflows.
Classification and types of Auto analyzers
Auto analyzers are classified based on the Operational Principles:
Sequential Flow Analyzers.
· Single/double channel Sequential Flow Analyzers.
· Multi-channel Sequential Flow Analyzers.
Discrete Analyzers.
· Semi-automatic discrete analyzers
· Fully automated descrete analyzer
i. Batch automated discrete analyzer
ii. Random access discrete analyzers
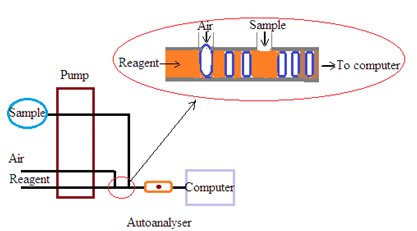
SEQUENTIAL FLOW ANALYZERS (CONTINUOUS FLOW ANALYZERS).
Single/double channel Sequential Flow Analyzers.
Single-channel Sequential Flow Analyzers (SFA) are automated instruments used to perform sequential chemical analyses of samples. These auto analyzers utilize a continuous flow system. The samples and reagents are continuously injected into a chamber for analysis. They are designed to automate various analytical processes such as mixing, dilution, and detection of analytes. The following is a single-channel Sequential Flow Analyzer parts structure and functions:
1. Sample Introduction System:
Sample Pump: It draws the sample from a sample cup or vial and introduces it into the system.
Sample Probe: A device for extracting a precise volume of sample from the sample cup.
Sample Tubing: Transports the sample through the system.
2. Reagent Introduction System:
Reagent Pump(s): Deliver precise volumes of reagents into the system for mixing with the sample.
Reagent Probe(s): Extract reagents from their respective containers.
Reagent Tubing: Transports reagents to the mixing coil.
Mixing Coil or Reaction Coil: The sample and reagents are mixed thoroughly to initiate chemical reactions if required.
Detection System:
Detector: Detects the analyte(s) based on a specific analytical method. Common detectors include photometric detectors, fluorometric detectors, or electrochemical detectors.
Flow Cell: A transparent cell where the reaction occurs and is measured by the detector.
Optical System: Includes light sources and optical components necessary for the detection method used.
Waste Management System:
Waste Tubing: Collects waste generated during the analysis and transports it to a waste container.
Waste Pump: Disposes of waste efficiently.
Control System:
Controller: Manages the operation of pumps, valves, and other components.
Software Interface: Allows users to input parameters, control the instrument, and view results.
Data Acquisition System: Collects and processes data from the detector.
Wash System (Optional):
Wash Reagent: Used to clean the system between analyses.
Wash Pump: Delivers wash reagent to the system.
Wash Tubing: Transports wash reagent to the appropriate parts of the system.
Accessories:
Valves: Control the flow of samples, reagents, and waste.
Tubing and Fittings: Connect various components of the system.
Heating/Cooling System: Maintains the temperature of the system if required by the analytical method.
Discrete Analyzers
These autoanalyzers use separate sample cups or cuvettes for individual sample analysis. It allows greater flexibility in assay selection and sample throughput.
Structure of a typical discrete analyzer:
Sample Tray or Carousel: The sample tray holds individual samples in separate wells or containers. It can accommodate a large number of samples for sequential analysis.
Sample Probe: A robotic arm moves to each sample position, aspirating a precise volume of the sample for analysis. The probe transfers the sample to the reaction vessel.
Reagent Tray or Carousel: It contains the necessary reagents for the analysis. Multiple reagents may be loaded into separate compartments. It is suitable for different tests to be performed sequentially.
Reaction Vessel; Each sample and reagent combination is mixed and reacted within a reaction vessel. These vessels are disposable cuvettes or tubes. It minimizes cross-contamination between samples.
Mixing System: It ensures thorough mixing of samples and reagents within the reaction vessel. Mixing is achieved through agitation, vortexing, or other mechanical means.
Detection System: It utilizes photometric or spectroscopic methods to measure the absorbance or fluorescence of the reaction mixture. The detection system quantifies the analyte concentration based on the intensity of the measured signal.
Washing Station: The sample probe is washed after each analysis. It prevents carryover contamination between samples. The washing station typically uses a cleaning solution or water to rinse the probe.
Control Unit: The entire operation of the discrete analyzer is controlled by a computerized system. The control unit coordinates the movement of the sample probe, dispensing of reagents, timing of reactions, and data acquisition.
Data Management: The discrete analyzer stores and manages data generated during analyses. It may be capable of data export, storage, and integration with laboratory information management systems (LIMS).
Importance/Advantages of Automation in Biochemistry:
Increased Efficiency: Automation reduces the time and effort required for biochemical analyses. It allows for the processing of multiple samples at one time. This increases the laboratory efficiency.
Precision and Accuracy: Automated systems perform with high precision and accuracy. This reduces the risk of human error associated with manual handling of samples, pipetting, and measurement, leading to more reliable and reproducible results.
Standardization: Automation standardizes laboratory procedures by minimizing sample processing and analysis variations. This ensures that results obtained from different batches of samples or different laboratories are comparable. It enhances the reliability of diagnostic tests and research findings.
Workflow Optimization: Automated biochemistry systems are attached to laboratory information management systems (LIMS). This rationalizes workflow management, sample tracking, and data management. This integration improves overall laboratory efficiency and facilitates better organization and traceability of experimental data.
Cost-effectiveness: The initial investment in automation technology is high. However, the long-term benefits of increased productivity, reduced labor costs, and improved resource utilization make it cost-effective. Laboratories handle larger volumes of samples with less staff. This leads to cost savings in the long run.
Enhanced Safety: Automation minimizes the handling of hazardous chemicals and biohazardous materials by laboratory personnel. This reduces the risk of exposure to harmful substances and improves workplace safety.
Dr. Pramila Singh
